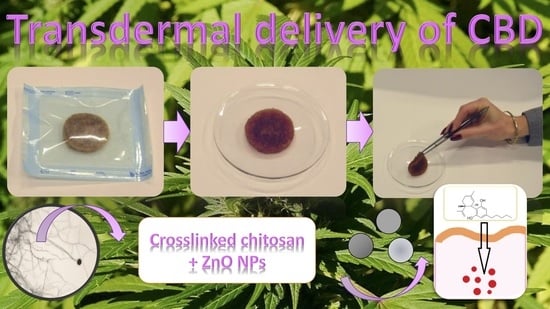
Development of Stimuli-Responsive Chitosan/ZnO NPs Transdermal Systems for Controlled Cannabidiol Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Physicochemical Properties Study
2.2.3. Drug Release Study
2.2.4. Cytotoxicity Study
3. Results and Discussion
3.1. Physicochemical Properties Study
3.2. Morphology Study
3.3. Swelling Abilities Study
3.4. Mechanical Properties and Water Permeation Study
3.5. Cannabidiol Controlled Release Study
3.6. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauss, G.L.; Sperling, M.R. Treating patients with medically resistant epilepsy. Neurol. Clin. Pract. 2011, 1, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, T.E.; Szaflarski, M.; Hansen, B.; Bebin, E.M.; Jerzy, P.; Szaflarski, J.P. Quality of life in adults enrolled in an open-label study of cannabidiol (CBD) for treatment-resistant epilepsy. Epilepsy Behav. 2019, 95, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Martin, R.C.; Grayson, L.P.; Ampah, S.B.; Cutter, G.; Szaflarski, J.P.; Bebin, E.M. Cognitive function and adaptive skills after a one-year trial of cannabidiol (CBD) in a pediatric sample with treatment-resistant epilepsy. Epilepsy Behav. 2020, 111, 107299. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.S.; Ajrawat, P.; Yang, Y.; Clarke, H. Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence. Molecules 2020, 25, 4042. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef]
- Silvestro, S.; Schepici, G.; Bramanti, P.; Mazzon, E. Molecular Targets of Cannabidiol in Experimental Models of Neurological Disease. Molecules 2020, 25, 5186. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharm. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Filippis, D.; Iuvone, T.; Blasio, A.; Steardo, A.; Esposito, G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 2009, 23, 597–602. [Google Scholar]
- Grotenhermen, F. Cannabinoids for therapeutic use. Designing systems to increase efficacy and reliability. Am. J. Drug Deliv. 2004, 2, 229–240. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, B.K.; Gloss, D.; Devinsky, O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav. 2017, 70, 341–348. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol—Transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2004, 93, 377–387. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Nair, S.S. Chitosan-based transdermal drug delivery systems to overcome skin barrier functions. J. Drug Deliv. Ther. 2019, 9, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan—Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, S.J.; Song, J.G.; Park, S.N. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Prinz, G.; Powell, J.; Greenlee, L.; Almodovar, J. Design, Characterization, and Modeling of a Chitosan Microneedle Patch for Transdermal Delivery of Meloxicam as a Pain Management Strategy for Use in Cattle. Mater. Sci. Eng. C 2021, 118, 111544. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Jacob, M.; Vignesh, N.S.; Varalakshmi, P. Pristine and modified chitosan as solid catalysts for catalytic and biodiesel production: A minireview. Int. J. Biol. Macromol. 2020, in press. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Stevens, W.F. Transdermal delivery controlled by a chitosan membrane. Drug Dev. Ind. Pharm. 2004, 30, 397–404. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Matysek, D. ZnO nanorods functionalized with chitosan hydrogels crosslinked with azelaic acid for transdermal drug delivery. Colloids Surf. B 2020, 194, 111170. [Google Scholar] [CrossRef]
- Kumaresapillai, N.; Basha, R.A.; Sathish, R. Production and Evaluation of Chitosan from Aspergillus Niger MTCC Strains. Iran. J. Pharm. Sci. 2011, 10, 14–23. [Google Scholar]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Hong, R. Large scale synthesis of ZnO nanoparticles via homogeneous precipitation. J. Cent. South Univ. 2012, 19, 863–868. [Google Scholar] [CrossRef]
- Ludi, B. Niederberger, Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalton Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef] [PubMed]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zheng, X.; Xu, Z.; Yi, C. ZnO-based nanocarriers for drug delivery application: From passive to smart strategies. Int. J. Pharm. 2017, 534, 190–194. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Dorado, J.; Almendros, G.; Field, J.A.; Sierra-Alvarez, R. Infrared spectroscopy analysis of hemp (Cannabis sativa) after selective delignification by Bjerkandera sp. at different nitrogen levels. Enzyme Microb. Technol. 2001, 28, 550–559. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125607. [Google Scholar] [CrossRef]
- Mikolaszek, B.; Kazlauske, J.; Larsson, A.; Sznitowska, M. Controlled Drug Release by the Pore Structure in Polydimethylsiloxane Transdermal Patches. Polymers 2020, 12, 1520. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, O.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [PubMed] [Green Version]















| Sample | CS, g | L-Asp; L-Glu, g | ZnO NPs, wt.% | MW Power, W | Time, min |
|---|---|---|---|---|---|
| hydrogel | 0.5 | 0.5;0.5 | 0.0 | 800 | 3 |
| CS-ZnO-1 | 0.5 | ||||
| CS-ZnO-2 | 1.0 | ||||
| CS-ZnO-3 | 1.5 | ||||
| CS-ZnO-4 | 2.0 | ||||
| CS-ZnO-5 | 2.5 | ||||
| CS-ZnO-6 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Szajna, E.; Matýsek, D.; Bogdał, D. Development of Stimuli-Responsive Chitosan/ZnO NPs Transdermal Systems for Controlled Cannabidiol Delivery. Polymers 2021, 13, 211. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020211
Radwan-Pragłowska J, Janus Ł, Piątkowski M, Sierakowska A, Szajna E, Matýsek D, Bogdał D. Development of Stimuli-Responsive Chitosan/ZnO NPs Transdermal Systems for Controlled Cannabidiol Delivery. Polymers. 2021; 13(2):211. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020211
Chicago/Turabian StyleRadwan-Pragłowska, Julia, Łukasz Janus, Marek Piątkowski, Aleksandra Sierakowska, Ernest Szajna, Dalibor Matýsek, and Dariusz Bogdał. 2021. "Development of Stimuli-Responsive Chitosan/ZnO NPs Transdermal Systems for Controlled Cannabidiol Delivery" Polymers 13, no. 2: 211. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020211