Copolyamide–Clay Nanotube Polymer Composite Nanofiber Membranes: Preparation, Characterization and Its Asymmetric Wettability Driven Oil/Water Emulsion Separation towards Sewage Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Copolyamide/HA(COHA) Nanocomposite Fiber
2.3. Transmission Electron Microscopy (TEM)
2.4. Scanning Electron Microscopy (SEM)
2.5. Surface Analysis Using Optical Microscopy
2.6. Fourier Transformed Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD) Analysis
2.8. Thermogravimetric Analysis (TGA)
2.9. Broadband Dielectric Spectroscopy (BDS)
2.10. UV-Visible Spectroscopy
2.11. Contact Angle Analysis
2.12. Optical Microscopy
2.13. Porosity Calculation
2.14. Oil-in-Water Emulsion Separation Experiment
3. Results and Discussion
3.1. Characterizations of Copolyamide-HA Nanocomposite Nanofiber Membranes
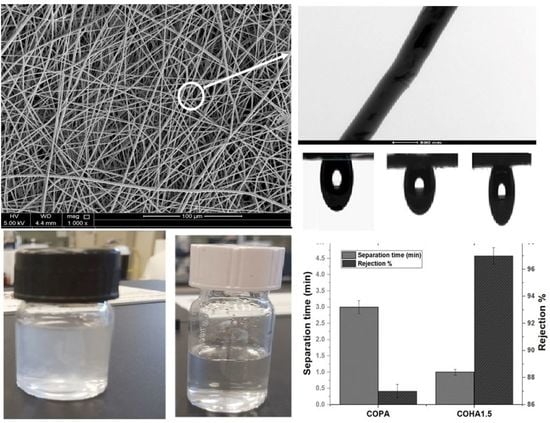
3.1.1. Scanning Electron Microscope (SEM) Analysis
3.1.2. Transmission Electron Microscopy (TEM) Analysis
3.1.3. Profilometry Analysis
3.1.4. Fourier Infrared Spectroscopy (FTIR) Analysis
3.1.5. X-ray Diffraction Analysis
3.1.6. Thermogravimetric Analysis (TGA)
3.1.7. Dielectric Spectroscopy Analysis
3.1.8. Contact Angle Analysis
3.2. Oil/Water Emulsion Separation Experiment
4. Conclusions
- Electrospun polymer composite membranes with uniform fiber morphology were synthesized using copolyamide as the polymer matrix and halloysite clay nanotubes as inorganic nanofillers by the simple electrospinning technique, without any further chemical modifications of the components.
- The addition of clay nanotubes improved the morphological, thermal, dielectric properties and liquid wettability properties of the polymer.
- The contact angle values of COHA1.5 for ethylene glycol, formamide, water and corn oil in air were 58 °C,110 °C, 120 °C and 2 °C, respectively, while those of COPA were 5 °C, 7 °C, 60 °C and 10 °C. The underwater oil contact angle of COPA was 128 ± 9 °C, while that of COHA1.5 improved to 136 °C ± 4 °C.
- The COHA1.5 showed an oil removal capacity of 97%, compared to the 87% by neat COPA. The water permeation capability of the COPA was 2087 L/m2 h, whereas COHA1.5 was found to be 6265 L/m2 h.
- The as-synthesized COPA–halloysite clay composite electrospun membranes showed asymmetric wettability, which in turn, helped the directional liquid transport, thereby decreasing the separation time of water from the oil/water emulsion by up to three times faster, and the rejection performance of composite fiber was 10% higher than the neat copolyamide membrane.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Li, K.; Ju, J.; Xue, Z.; Ma, J.; Feng, L.; Gao, S.; Jiang, L. Structured cone arrays for continuous and effective collection of micron-sized oil droplets from water. Nat. Commun. 2013, 4, 2276. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.J.; Zhang, M.J.; Liu, Z.C.; Huang, C.B.; Fu, G.D. Nature-inspired creation of a robust free-standing electrospun nanofibrous membrane for efficient oil-water separation. Environ. Sci.-Nano 2018, 2, 2909–2920. [Google Scholar] [CrossRef]
- Wang, X.F.; Yu, J.Y.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Trinh, D.X.; Tran, T.P.N.; Taniike, T. Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration. Sep. Purif. Technol. 2017, 177, 249–256. [Google Scholar] [CrossRef]
- Wang, H.; Gao, F.; Ren, R.; Wang, Z.; Yue, R.; Wei, J.; Zhang, X. Caffeic acid polymer rapidly modified sponge with excellent anti-oil-adhesion property and efficient separation of oil-in-water emulsions. J. Hazard. Mater. 2021, 404, 124197. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Anis, B.; Khalil, A.S. Facile surface treatment and decoration of graphene-based 3D polymeric sponges for high performance separation of heavy oil-in-water emulsions. J. Environ. Chem. Eng. 2021, 9, 105087. [Google Scholar] [CrossRef]
- Ge, J.; Zong, D.; Jin, Q.; Yu, J.; Ding, B. Biomimetic and superwettable nanofibrous skins for highly efficient separation of oil-in-water emulsions. Adv. Funct. Mater. 2018, 28, 1705051. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of superwetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Song, C.; Rutledge, G.C. Electrospun polyimide fiber membranes for separation of oil-in-water emulsions. Sep. Purif. Technol. 2021, 270, 118825. [Google Scholar] [CrossRef]
- Awang, N.; Nasir, A.M.; Yajid, M.A.M.; Jaafar, J. A Review on Advancement and Future Perspective of 3D Hierarchical Porous Aerogels Based on Electrospun Polymer Nanofibers for Electrochemical Energy Storage Application. J. Environ. Chem. Eng. 2021, 105437. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Photo-Fenton assisted self-cleaning hybrid ultrafiltration membranes with high-efficient flux recovery for wastewater remediation. Sep. Purif. Technol. 2020, 249, 117159. [Google Scholar] [CrossRef]
- Choong, L.T.S.; Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions using electrospun fiber membranes and modeling of the fouling mechanism. J. Membr. Sci. 2015, 486, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, C.; Zhao, G.; Li, C.; Liu, L.; Yu, J.; Jiao, F. Electrospun composite membrane with superhydrophobic-superoleophilic for efficient water-in-oil emulsion separation and oil adsorption. Colloids Surf. A Physiochem. Eng. Asp. 2020, 602, 125158. [Google Scholar] [CrossRef]
- Hu, L.; Gao, S.; Zhu, Y.; Zhang, F.; Jiang, L.; Jin, J. An ultrathin bilayer membrane with asymmetric wettability for pressure responsive oil/water emulsion separation. J Mater. Chem. A 2015, 3, 23477–23482. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Ultra-wetting graphene-based PES ultrafiltration membrane–a novel approach for successful oil-water separation. Water Res. 2016, 103, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Gao, L.; Hou, Y.; Liu, Z.; Jiang, L. Temperature controlled water/oil wettability of a surface fabricated by a block copolymer: Application as a dual water/oil on–off switch. Adv. Mater. 2013, 25, 273–277. [Google Scholar] [CrossRef]
- Moghaddasi, A.; Sobolčiak, P.; Popelka, A.; Krupa, I. Separation of Water/Oil Emulsions by an Electrospun Copolyamide Mat Covered with a 2D Ti3C2Tx MXene. Materials 2020, 13, 3171. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; All Consulting, LLC: Tulsa, OK, USA, 2005; 53p. [Google Scholar]
- Li, S.; He, Y.; Zhang, L.; Li, J.; Nie, Y.; Li, H.; Bai, Y. Designing nanofibrous membrane with biomimetic caterpillar-like structured for highly-efficient and simultaneous removal of insoluble emulsified oils and soluble dyes towards sewage remediation. J Hazard. Mater 2021, 414, 125442. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.J.; Kim, Y.H. Improvement of interfacial adhesion of incorporated halloysite-nanotubes in fiber-reinforced epoxy-based composites. Appl. Sci. 2017, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Lazzara, G. Ecocompatible halloysite/cucurbit [8] uril hybrid as efficient nanosponge for pollutants removal. ChemistrySelect 2016, 1, 1773–1779. [Google Scholar] [CrossRef]
- Song, Q.; Wang, H.; Han, S.; Wang, J.; Zhang, B.; Zhang, Y. Halloysite nanotubes functionalized cotton fabric for oil/water separation. Prog. Org. Coat. 2020, 148, 105839. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Liu, S.; Gao, J.; Lang, J.; Li, C.; Yan, Y. Facile preparation of halloysite nanotube-modified polyvinylidene fluoride composite membranes for highly efficient oil/water emulsion separation. J. Mater. Sci. 2019, 54, 8332–8345. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic durable multifunctional self-cleaning nanofibrous membrane with outstanding oil/water separation, photodegradation of organic contaminants, and antibacterial performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Liang, Y.; Crawford, A.; Coates, P.; Chen, D.; Zhang, L. Fabrication and evaluation of electrospun PCL–gelatin micro-/nanofiber membranes for anti-infective GTR implants. J. Mater. Chem. B 2014, 2, 6867–6877. [Google Scholar] [CrossRef]
- Ndesendo, V.M.; Choonara, Y.E.; Meyer, L.C.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Pillay, V. In vivo evaluation of a mucoadhesive polymeric caplet for intravaginal anti-HIV-1 delivery and development of a molecular mechanistic model for thermochemical characterization. Drug Dev. Ind. Pharm. 2015, 41, 1274–1287. [Google Scholar] [CrossRef]
- Vinokurov, V.; Stavitskaya, A.; Glotov, A.; Ostudin, A.; Sosna, M.; Gushchin, P.; Lvov, Y. Halloysite nanotube-based cobalt mesocatalysts for hydrogen production from sodium borohydride. J. Solid State Chem. 2018, 268, 182–189. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Tang, W.; Ma, R. Facile synthesis and characterization of ZnO nanoparticles grown on halloysite nanotubes for enhanced photocatalytic properties. Sci. Rep. 2017, 7, 2250. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutynski, M.; Soltys, J.; Pytlinski, A. Purification of halloysite by magnetic separation. Physicochem. Probl. Miner. 2016, 52, 991–1001. [Google Scholar]
- Lisuzzo, L.; Wicklein, B.; Dico, G.L.; Lazzara, G.; Del Real, G.; Aranda, P.; Ruiz-Hitzky, E. Functional biohybrid materials based on halloysite, sepiolite and cellulose nanofibers for health applications. Dalton. Trans. 2020, 49, 3830–3840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with -aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Sunitha, V.R.; Radhakrishnan, S. Impedance and dielectric studies of nanocomposite polymer electrolyte systems using MMT and ferroelectric fillers. Ionics 2016, 22, 2437–2446. [Google Scholar] [CrossRef]
- Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Highly dispersed polyamide-11/halloysite nanocomposites: Thermal, rheological, optical, dielectric, and mechanical properties. J. Appl. Polym. Sci. 2013, 130, 313–321. [Google Scholar] [CrossRef]
- Zhu, T.; Qian, C.; Zheng, W.; Bei, R.; Liu, S.; Chi, Z.; Xu, J. Modified halloysite nanotube filled polyimide composites for film capacitors: High dielectric constant, low dielectric loss and excellent heat resistance. RSC Adv. 2018, 8, 10522–10531. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Wang, S.T.; Wei, Z.X.; Song, Y.L.; Jiang, L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Liu, T.Y.L.; Chen, Z.Y.; Kim, C.J. A dynamic cassie-baxter model. Soft Matter. 2015, 11, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Sample Name | HA% | Viscosity of Solution (Pa × s) | Fiber Diameter (nm) | Sa (µm) | Porosity (%) | Average Pore Size (nm) |
|---|---|---|---|---|---|---|
| COPA | 0 | 1.41 | 966 ± 200 | 3.4 | 78.1 | 701 ± 110 |
| COHA 0.5 | 0.5 | 1.79 | 1265 ± 152 | 4.5 | 81.2 | 756 ± 116 |
| COHA1 | 1 | 1.81 | 1349 ± 250 | 5.8 | 83.5 | 810 ± 212 |
| COHA1.5 | 1.5 | 1.84 | 1040 ± 145 | 6.6 | 85.3 | 840 ± 227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhagyaraj, S.; Sobolčiak, P.; Al-Ghouti, M.A.; Krupa, I. Copolyamide–Clay Nanotube Polymer Composite Nanofiber Membranes: Preparation, Characterization and Its Asymmetric Wettability Driven Oil/Water Emulsion Separation towards Sewage Remediation. Polymers 2021, 13, 3710. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13213710
Bhagyaraj S, Sobolčiak P, Al-Ghouti MA, Krupa I. Copolyamide–Clay Nanotube Polymer Composite Nanofiber Membranes: Preparation, Characterization and Its Asymmetric Wettability Driven Oil/Water Emulsion Separation towards Sewage Remediation. Polymers. 2021; 13(21):3710. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13213710
Chicago/Turabian StyleBhagyaraj, Sneha, Patrik Sobolčiak, Mohammad A. Al-Ghouti, and Igor Krupa. 2021. "Copolyamide–Clay Nanotube Polymer Composite Nanofiber Membranes: Preparation, Characterization and Its Asymmetric Wettability Driven Oil/Water Emulsion Separation towards Sewage Remediation" Polymers 13, no. 21: 3710. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13213710