3.1. Full Factorial Design Analysis
The selection of the processing temperature
P, mold temperature
M, screw speed
S, and cooling time
C in the FFD is based on other PHA studies [
33,
41,
42,
43], preliminary DSC results, and practical considerations from an extensive pre-screening study. This selection of the processing parameters is important as mold and processing temperature are known to influence the mechanical properties of melt-processed bioplastics [
44,
45,
46].
Table 4 shows a detailed summary of all executed test runs of the FFD matrix with factors and mechanical properties as a response. The average value of three sample measurements is used for calculations (
n = 3). Large differences in elongation at break values are apparent between individual test runs. The maximum difference in elongation at break amounts to 263% elongation between individual runs nr. 1 and nr. 5. The Young’s modulus varies between 872 MPa (run 15) and 1084 MPa (run 2) and the tensile strength ranges between 20.6 MPa (run 15) and 22.4 MPa (run 2).
Figure 3 shows the main effects plots for tensile strength, elongation at break, and Young’s modulus, i.e., the mean response value at each factor level (+, 0, −) [
36]. Main effects plots are a relative measure of factor significance on the system response. If the line from low to high factor levels is steep, factors have a high effect on the response. On the contrary, if the line is nearly horizontal, factors have a limited effect on the response. The sign of main effects relates to the direction of the line on the graph, showing a decrease or increase of the average response value. The center points in the main effects plot have to be interpreted with care because they are based on only one center point run (run 17). The main effects are calculated following Equation (8) [
36]:
where
is the main effect of parameter X on the studied response,
is the average response at high-level factor setting, and
is the average response at a low-level factor setting.
From
Figure 3, it can be seen that processing temperature
P has the highest influence on tensile strength. Lower processing temperatures result in higher average tensile strength values (negative main effect). The main effect of processing temperature
on tensile strength equals −0.59 MPa. The effect of mold temperature
M on tensile strength is lower and equals −0.34 MPa. The influence of screw speed
S and cooling time
C on tensile strength is more limited (
= −0.19 MPa and
= +0.26 MPa). It is also apparent from
Figure 3 that mold temperature
M has the highest impact on elongation at break. High mold temperatures result in extensively increased elongation at break values. The main effect of mold temperature on elongation at break (
equals +175%. On the contrary, the effect of processing temperature
P, screw speed
S, and cooling time
C is limited (
= −10%,
= +21% and
= +8%). Further, an increased mold temperature
M results in lower average Young’s modulus values (negative main effect). The main effect of mold temperature on Young’s modulus
is −110 MPa. The effect of processing temperature
P (
= −33 MPa) is lower, while the effect of screw speed
S (
= −9 MPa) and cooling time
C (
= +9 MPa) are limited.
3.2. Effect of Processing Parameters on TS, ε, and E
Predictive models to estimate TS, ε, and E, are developed after statistical analysis in JMP
® Pro by selecting significant parameters and interactions (
p < 0.05).
Table 5,
Table 6 and
Table 7 show the terms, parameter estimates, standard error, and t-ratio of the TS, ε, and E modeling respectively if the
p-value is below 0.05 in the backward stepwise regression method.
Table 5 shows that the main factors processing temperature
P and mold temperature
M have a significant effect on tensile strength. No main factor interactions are present. Optimal settings for maximum tensile strength (MPa) values are calculated with the following predictive Equation (9):
The intended goal is to increase the strength of the material. Therefore, maximized tensile strength is predicted at processing temperature P = 145 °C and mold temperature M = 40 °C and amounts to 21.70 MPa, within a 95% confidence interval (CI) of 21.44 MPa and 21.96 MPa.
Our findings of higher TS of PHBHHx samples at lower processing temperature
P are not in total agreement with the results on PHBV processing of Vandi et al. [
47]. In contrast to our results, they observed a lower TS of PHBV using a combination of low processing temperature
P and low screw speed
S and attributed this to an incomplete melt consolidation of the material. However, a combination of high processing temperature
P > 190 °C and high screw speed
S > 150 rpm also resulted in decreased tensile strength, similar to our results. This was attributed to a lower melt consolidation pressure at the extruder die due to higher melt flows when processing at higher temperatures. A decrease in PHBV mechanical performance at higher process temperatures was also reported elsewhere [
33].
An explanation for a decrease in tensile strength at elevated process temperatures could be found in the fact that PHBHHx twin-screw extrusion and injection molding at high temperatures can lead to a reduction in molecular weight due to thermal degradation [
18]. Random chain scission has been reported as the degradation mechanism causing a rapid decrease in molecular weight of PHAs during thermal treatment [
46]. The theoretical processing window of the PHBHHx granulate is determined as the temperature region between the melting temperature of 130.5 °C (DSC) and the onset degradation temperature of 279.5 °C (TGA) and should be indicative for suitable melt processing of the polymer. Processing at temperatures of
P = 145–160 °C seems suitable with a granulate peak degradation temperature of 295.3 °C (determined with TGA, and similar to the literature [
48]). Although the processing temperature
P was chosen well in the lower part of this theoretical range, the practical processing window is smaller because thermo-mechanical and thermo-oxidative degradation of the polymer might occur due to high shear (high rotational screw speed) at elevated temperatures [
49].
Thermal degradation was quantified in separate GPC (SEC) experiments as the loss in molecular weight after processing at different temperatures. The weight average molecular weight (M
w) and number average molecular weight (M
n) of the unprocessed pellet and samples processed at different temperatures are shown in
Table 8. The M
w after processing at 145 °C, 160 °C, 170 °C, and 180 °C decreases respectively with 13%, 23%, 19%, and 48%; the M
n does not decrease at
P = 145 °C but decreases with 3, 8 and 37% at
P = 160 °C, 170 °C, and 180 °C, respectively. This reduction in molecular weight might explain the decrease in TS when the temperature is increased (
Figure 3). Processing at 180 °C seems not suitable due to a severe decrease in the polymer molecular weight. A decrease of molecular weight of PHB and PHBV (in the range of 4–53%) at elevated temperatures was reported earlier [
50] and can decrease the tensile strength [
51]. Decreased tensile strength of PLA by reduction of molecular weight was partially attributed to a decrease in chain length and number of chain entanglements [
52]. Molecular weight decrease after processing at elevated temperatures was also reported for conventional PS and PMMA [
53].
Concerning the elongation at break modeling shown in
Table 6, the statistical analysis indicates that only the mold temperature
M has a significant effect on this property. No main factor interactions are present. Optimal settings for maximum elongation (%) values are calculated with the following predictive Equation (10):
The intended goal is to increase the elongation of the material. Therefore, maximized elongation is predicted at mold temperature M = 80 °C and amounts to 330%, within a 95% CI of 301% and 360%.
Young’s modulus is significantly affected by the main factors mold temperature
M and processing temperature
P (
Table 7). No main factor interactions are apparent. Young’s modulus values (MPa) are calculated with the following predictive Equation (11):
The intended Young’s modulus value of the material is dependent on the application. Therefore, maximized Young’s modulus is predicted at 145 °C processing temperature P and 40 °C mold temperature M, amounting to 1047 MPa (95% CI: 1026–1067 MPa). On the contrary, minimized Young’s modulus is predicted at 160 °C processing temperature P and 80 °C mold temperature M, amounting to 904 MPa (95% CI: 884–925 MPa).
3.3. Predictive Model Validation
The mathematical models described in the previous section highlight the selection of significant processing parameters and their effect on the separate mechanical properties. By combining these models, optimal PHBHHx processing conditions to maximize the elongation at break (while maintaining high tensile strength) are selected as: processing temperature P = 145 °C, mold temperature M = 80 °C, screw speed S = 50 rpm, and cooling time C = 60 s (settings A). An optimal mold temperature M of 80 °C is selected because the effect of a low mold temperature M = 40 °C on tensile strength is limited. Since long(er) cooling times of samples in the mold (300 s) lead to increased injection molding cycle times and lower productivity, an optimal cooling time C of 60 s is selected because of the influence of this parameter on mechanical properties—especially elongation at break—is limited. According to the model and for comparison, processing temperature P = 160 °C, mold temperature M = 40 °C, screw speed S = 50 rpm, and cooling time C = 60 s, are considered as non-optimal processing conditions resulting in lower elongation at break and tensile strength (settings B), while minimizing the injection molding cycle time.
The goal of the FFD and model development is to predict optimal processing conditions for maximized mechanical properties (i.e., the intended use), not to predict the total design space in detail. Therefore, only a validation of the models for both predicted settings A and B is performed. The validation of the predictive models is executed following the confidence interval confirmation approach (CICon). This approach includes the calculation of confidence intervals using the confirmation runs themselves, rather than the confidence intervals of the experimental runs [
36,
37]. If the predicted values of the mechanical properties fall within this confidence interval (CI), the model is considered appropriate. The predicted mechanical properties are validated at a tensile test speed of 20 mm/min for both melt processing settings and are shown in
Table 9. A 95% CI is used to assess the prediction quality of the model.
The predictive models show good estimations for elongation at break and Young’s modulus. For tensile strength, however, they show a slight underestimation for both test settings. The underestimations can be attributed to very small deviations of tensile strength in function of processing conditions, resulting in relatively small confidence intervals. Higher prediction accuracy for tensile strength can be achieved by adding repetitions, replications, or extra points to the design.
In addition, samples from both validation runs are also compared at a tensile test speed of 1 mm/min. As the model is built on tensile properties measured at 20 mm/min, no prediction values for 1 mm/min are available in
Table 9. Tensile testing at a lower speed (1 mm/min) shows similar results for Young’s modulus at a test speed of 20 mm/min. However, testing the PHBHHx samples at low speed shows lower values of tensile strength and elongation at break compared to higher speeds.
Referring to the first objective, this study shows that PHBHHx can be melt-processed in products with TS ranging between 20–22 MPa, ε ranging between 19–342%, and E ranging between 883–1205 MPa depending on the processing conditions and tensile test method. The tensile strength of PHBHHx is relatively low compared to semi-crystalline PLA (50–70 MPa) [
54], isotactic PP (29–39 MPa), PHB (40 MPa) [
12], PHBV (30–38 MPa) [
12] and LDPE (15–79 MPa) [
12]. The elongation at break of PHBHHx is high compared to semi-crystalline PLA (4%) [
54], PHBV (0.8–58%) [
55,
56,
57] and PHB (3–8%) [
12], and closer to ε values found for isotactic PP (500–900%) [
13] and LDPE (150–600%) [
12]. The Young’s modulus of PHBHHx is higher compared to LDPE (50–100 MPa) and in line with values found for isotactic PP (1000–1700 MPa) [
12], but lower as compared to semi-crystalline PLA (3000 MPa) [
54], PHBV (700–2900 MPa) [
12] and PHB (3500–4000) [
12]. In conclusion, the flexibility of PHBHHx can be adapted within the range of flexible packaging materials by an appropriate selection of processing conditions.
3.5. Influence of the Mold Temperature on Process-Induced Microstructure
The thermal and mechanical behavior of PHBHHx is strongly dependent on the crystal structure [
29,
32]. In order to investigate the observed impact of the mold temperature
M, on TS, ε, and E properties, DSC, POM, and XRD measurements are performed to characterize the developed crystallinity and microstructure during processing.
The DSC first heating curves of samples molded at temperatures of
M = 40–80 °C are shown in
Figure 6. The glass transition temperature (T
g) of all samples equals −1 to 1 °C (similar to the literature [
48,
58]). As no shift is observed, T
g is not shown in
Figure 6. Three endothermal peaks are apparent in this first heating curve. The small peak I at ±84–89 °C can be attributed to the melting of secondary crystallites formed during secondary crystallization/molding. Peak II at ±107–119 °C is due to the melting of primary lamellae [
16,
59]. Peak III is due to the melting of lamellae formed through thickening and reorganization during DSC heating [
16,
32]. It is apparent from
Figure 6 that the melting peak temperature of the primary lamellae (peak II) shifts to higher temperatures and the peak area increases when processed at elevated mold temperatures (less recrystallization during DSC). Allowing time to crystallize at higher temperatures in the mold leads to increased crystal sizes with higher melting points. In addition, the area of peak III decreases with increasing mold temperature, indicating a less exhaustive recrystallization process during heating. All peak temperatures are shown in
Table 10.
The calculation of the processing-induced crystalline content is based on the sum of enthalpies (ΔH
m) of the three endothermic peaks. These peaks hide the exothermic heat due to recrystallization, which is expected to be as large as the endothermic melting enthalpy of the recrystallized crystals. As a result, the full integral actually corresponds to the melting enthalpy of the processing induced primary and secondary crystals. The crystallinity remains constant with increasing mold temperature
M, around ±38–39% (
Table 10). These results differ from a study on PLA, where almost no crystallization was observed after molding at room temperature or up to 50 °C [
60,
61]. Crystal formation and enlargement in PLA was achieved by post-annealing at temperatures of 80–120 °C, with a maximal increase of elongation at break when annealing occurred at 80 °C for 0.5 and 2 h [
61]. As the mold temperature
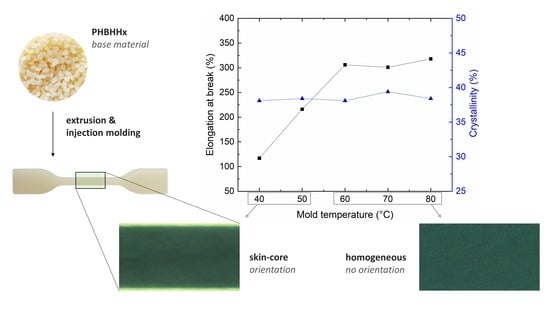
M has no effect on the crystallinity of the injection molded PHBHHx, other differences in microstructure, orientation effects, in particular, are investigated hereafter to account for the observed variations in mechanical properties.
Several studies have shown that melt processing parameters like mold temperature
M and processing temperature
P can induce order of magnitude variations in mechanical properties like elongation at break, due to microlayer development in the moldings [
62,
63,
64]. A conventional three-layer process-induced morphology has been reported for a range of materials (PP, polyamide, poly(butylene terephthalate)…), with different semi-crystalline characteristics between the skin, shear, and core layers [
65,
66,
67]. By increasing the mold temperature, the shear and skin layers reduce in thickness, providing more volume to the spherulitic core layer [
68,
69,
70]. Morphological analysis on PHBHHx samples produced at mold temperatures
M from 40 °C to 80 °C was performed in order to investigate the process-induced microstructure.
POM micrographs representing outer (I), middle (II), and inner (III) regions of a sample produced at
M = 40 °C are shown in
Figure 7. It can be seen that a distinctive skin-core layer structure is apparent, with high birefringence in the skin region and a gradual brightness decrease towards the core. The skin birefringence is an indication of molecular chain orientation [
71]. The birefringence is absent towards the core because the polymer chains are randomly oriented, becoming optically isotropic [
71]. The skin and shear layer are defined as regions A and B in
Figure 7 based on birefringence intensity. The thickness of the skin (A) and shear layer (B) are respectively ±35 µm (±1.75% of sample thickness) and ±65 µm (±3.25% of sample thickness) on both sides of the sample. Hence, the total skin-like region is approximately 10% of the sample thickness. No spherulites are visible in the core region (no Maltese crosses) because it might be that the thin films (40 µm) are thicker than the spherulite size.
POM of samples produced at mold temperature
M = 50 °C (
Figure 8), shows a decrease in the thickness of the skin layer to ±10 µm (±0.50% of sample thickness) on both sides of the sample, without a distinguishable transition layer towards the core. The skin-like region is reduced from 10% at
M = 40 °C to approximately 1% at
M = 50 °C, with respect to the total sample thickness. The other POM micrographs in
Figure 8 show that skin formation is absent at higher mold temperatures of
M = 60, 70, and 80 °C. The oriented polymer chains are relaxed at elevated mold temperatures prior to solidification, which reduces orientational birefringence [
71]. Hence, these moldings are characterized by a homogeneous and less oriented microstructure throughout the whole sample. At
M = 80 °C, spherulites are visible because their size is increased compared to samples at
M = 40 °C.
XRD data were obtained in transmission through the central part of the tensile test bar. As a result, these patterns are superpositions of the scattering coming from the bulk as well as the surface of the samples. The 2D scattering patterns in
Figure 9 (left side) of
M = 40 °C and 80 °C show typical PHB reflections with Miller indices 020, 110, 031, and 040 [
72]. The individual scattering angle (
) scans along
φ directions of 0°, 45°, 90°, 135°, and 180° (
Figure 9, right side) are compared to the azimuthally averaged patterns (full lines) obtained by averaging the intensities over al
φ directions. The injection molding flow direction is parallel to the meridional direction (
φ = 0°).
For
M = 80 °C (
Figure 9A), the scans over the different
φ directions yield intensities equal to those of the azimuthal average. This means that no orientation is apparent.
For
M = 40 °C (
Figure 9B), the SAXS peaks are shifted to larger scattering angles compared to
M = 80 °C, implying that the crystal-to-crystal separation for
M = 40 °C is shorter. As the (DSC based) crystallinity for
M = 40 °C is equal to that of
M = 80 °C, one can conclude that the crystals for
M = 40 °C, are thinner. No efforts were made to further quantify the size differences. The reflections in the WAXD part have not shifted drastically and peak intensities are comparable, indicative of a similar crystallinity as in samples with
M = 80 °C and confirming the presented DSC data. The circled intensities of the angular scans (
Figure 9B, right) are larger than the azimuthal average and point at (partial) molecular orientation along the injection axis (
φ = 0°). It can be seen that the 020 and 110 reflections are stronger along the equator, meaning that a fraction of the chains (logically residing in the skin layer) are oriented parallel to the injection molding direction. However, the crystalline lamellar stacks are oriented perpendicular to this direction because the SAXS intensity is stronger along the meridional. This scattering behavior is typical of shish kebab structures as, e.g., observed earlier for polyethylene, which also has an orthorhombic crystal structure [
73]. The orthogonal relation between the WAXD and SAXS reflections furthermore indicates that the chains within the crystals are oriented perpendicular to the lamellar surface direction.
It can qualitatively be derived from the scattering pattern that the orientation for
M = 40 °C is not very large. This corresponds well with the POM finding that orientation is limited to the skin. The scattering from the core is isotropic, comparable to
M = 80 °C. To quantify the degree of molecular orientation with respect to the injection direction (given by
φ = 0), orientation functions
were derived from the
φ dependent intensity of the 110 reflection. Intensities are obtained by integrating the 110 peaks after separating them from the patterns using straight sectors. To illustrate the integration procedure, the areas of the 110 reflections for
equal to 0, 45, and 90 °C, are color shaded in
Figure 9A. The values of the orientation function
in relation to mold temperature
M, tensile properties and crystallinity are shown in
Table 11. Zero values for
are obtained when the crystals and the chains they contain are oriented randomly and values of 1 correspond to perfect alignment with respect to the injection melt flow direction [
74]. The molecular orientation is significant for
M = 40 °C and 50 °C, but very small for the other mold temperatures
M = 60–80 °C. The as-obtained molecular orientation parameter
only relates to the crystalline parts of the material. The overall molecular orientation is presumably less than given by
because amorphous matter more readily randomizes. This difference may be more important for
M = 50 °C compared to
M = 40 °C, as the POM experiments clearly indicate that the overall orientation in the skin is less developed for
M = 50 °C. The increased molecular orientation at low mold temperature occurs due to polymer chain molecules being aligned, sheared, and stretched in the direction of flow during the injection. The frozen orientation is more retained at faster cooling rates of the polymer melt [
75], i.e., at lower mold temperatures. The orientation effect is highly pronounced in polymer regions near the mold wall, where molecules are frozen in their stretched state, forming a skin-like layer. Taken together, the XRD and POM results suggest that processing at low mold temperature (
M = 40 °C) induces skin formation and increased molecular orientation in the sample, while the latter is minimized at higher mold temperatures (
M = 80 °C), i.e., at slow cooling rates.
The results of the microstructural analysis from DSC, POM, and XRD measurements indicate that the mold temperature changes the crystallization and orientation conditions of the melt during processing. Molding at low mold temperature (40 °C) produces small crystals with high molecular orientation in a skin-like structure comprising approximately 10% of the sample thickness. Due to the stretching and shearing of the polymer chains during injection molding, the inherent maximum stretch and strength of the chains are nearly reached compared to their initial relaxed state [
75]. This orientation results in slightly increased tensile strength and Young’s modulus but highly reduced elongation at break. On the contrary, the selected optimal processing condition (A) with higher mold temperature (80 °C) gives rise to slower cooling and a suitable time interval for the polymer chains to relax before solidification by crystallization, compared to molding at a lower temperature. Processing of PHBHHx at higher mold temperature reduces process-induced skin formation and molecular orientation. The produced samples have increased crystal size (derived from DSC and qualitatively from SAXS) and a homogeneous microstructure throughout the sample thickness (derived from POM). This microstructure results in increased elongation at break values because the randomly curled and oriented polymer chains can be stretched to a further extent [
76]. In addition, increased elongation values at
M = 80 °C are also likely to be related to lower residual stress of the polymer chains induced by slower cooling rates of the melt during molding [
71,
77].
In this study, a higher mold temperature was found to cause improved elongation at break due to a reduction of molecular orientation and skin formation. The increased elongation at break was not attributed to changes in the overall crystallinity. This finding is contrary to previous studies which have suggested that higher mold temperatures result in lower elongation at break, mainly due to the formation of larger spherulites and increased crystallinity. Fast cooling (quenching) of the PHB melt is related to the formation of smaller crystallites, while slow cooling results in larger spherulites [
31]. These large spherulites are more brittle, giving rise to poor mechanical properties like short elongation and low impact strength. Increased crystallinity at elevated mold temperatures was reported for PLA [
45] and isotactic PP [
78,
79], leading to decreased elongation at break and impact properties. This negative effect on mechanical properties was attributed to a volumetric increase of more brittle/rigid crystalline regions and a decrease of loosely arranged amorphous regions, causing brittle instead of ductile failure.
Several other attempts to improve the mechanical properties of PHAs were made previously by incorporating for example fillers and plasticizers. The addition of plasticizers in PHB can improve the elongation at break with values up to 45% but at the expense of tensile strength and Young’s modulus [
17]. Some talc-filled PHBHHx compounds remain very brittle with elongation values under ±5% [
18], while the addition of
L-phenylalanine [
19] and ultrafine talc [
20] nucleating agents have almost no effect on elongation at break. The limited increase of mechanical properties, i.e., elongation at break for PHAs in these studies highlights the importance of process optimization, as discovered in this work. As shown here, elongation at break values can be increased extensively with average values up to ±175% by optimizing process conditions. However, increasing the crystallization rate and nucleation by incorporation of fillers and the combination of efficient and optimal processing conditions can possibly improve and modify the mechanical properties of PHBHHx to a further extent. In addition, PHBHHx post-annealing can be a valuable approach to promote crystallization and improve mechanical properties, as previously shown for PLA [
80].