Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Plain Cyclodextrin DPC Cross-Linked Nanosponge
2.2.2. Preparation of the Drug-Loaded Nanosponges
2.2.3. Curcumin-β-Cyclodextrin Complex
2.2.4. Corresponding Physical Mixtures
2.2.5. HPLC Analysis of Curcumin Concentration
2.2.6. Phase Solubility Studies
2.2.7. Molecular Modeling Studies
2.2.8. Physicochemical Evaluation of Curcumin-β-Cyclodextrin Complex and Curcumin-Loaded Nanosponge
Particle Size Distribution and Polydispersity Index
Zeta Potential
The Powder Diffraction Pattern (PXRD)
Differential Scanning Calorimetry (DSC)
Fourier-Transform Infrared (FTIR) Spectroscopy
In Vitro Release Studies
Scanning Electron Microscopy (SEM)
2.2.9. Statistical Analysis
3. Results and Discussion
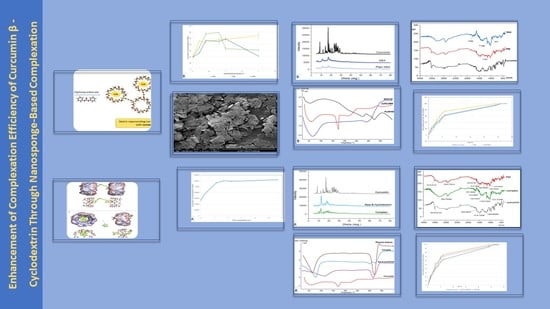
3.1. Phase Solubility Studies
3.2. Molecular Modeling
3.3. Zeta Potentials and Particle Size Distribution
3.4. The Powder Diffraction Pattern (PXRD)
3.5. Differential Scanning Calorimetry (DSC)
3.6. Fourier-Transform Infrared Spectroscopy
3.7. In Vitro Release Experiments
3.8. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almagro, E.Q.; Bosca, A.R.; Bernd, A.; Zapata, J.P.; Joaquin, D.; Mira, D.P.; Gutierrez, M.A.C.; Ortells, J.M.S. Pharmacological Activities of Curcuma Longa Extracts. U.S. Patent US7220438B2, 2007. Available online: https://patents.google.com/patent/US7220438B2/en (accessed on 12 November 2021).
- Yadav, D.; Yadav, S.K.; Khar, R.K.; Mujeeb, M.; Akhtar, M. Turmeric (Curcuma longa L.): A promising spice for phytochemical and pharmacological activities. Int. J. Green Pharm. (IJGP) 2013, 7, 85. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.D.; Chen, X.J.; Hu, Y.H.; Yu, Z.J.; Wang, D.; Liu, J.Z. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother. Res. 2013, 27, 422–430. [Google Scholar] [CrossRef]
- Chen, D.; Dai, F.; Chen, Z.; Wang, S.; Cheng, X.; Sheng, Q.; Lin, J.; Chen, W. Dimethoxy curcumin induces apoptosis by suppressing survivin and inhibits invasion by enhancing E-cadherin in colon cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, S.; Wang, Y.; Yu, Z.; Guan, K.; Kan, Q. Curcumin induces apoptosis and inhibits proliferation in infantile hemangioma endothelial cells via downregulation of MCL-1 and HIF-1α. Medicine 2018, 97, e9562. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Yang, J.; Li, H.; Zhang, H.; Zheng, P. Curcumin induces apoptotic cell death and protective autophagy in human gastric cancer cells. Oncol. Rep. 2017, 37, 3459–3466. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Endo, H.; Inoue, I.; Masunaka, K.; Tanaka, M.; Yano, M. Curcumin induces apoptosis in lung cancer cells by 14-3-3 protein-mediated activation of Bad. Biosci. Biotechnol. Biochem. 2020, 84, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit s100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okagu, O.D.; Verma, O.; McClements, D.J.; Udenigwe, C.C. Utilization of insect proteins to formulate nutraceutical delivery systems: Encapsulation and release of curcumin using mealworm protein-chitosan nano-complexes. Int. J. Biol. Macromol. 2020, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Al-Debsi, T.; Al-Shalabi, E.; Hasan Ibrahim, L.; Faruqu, F.N.; Walters, A.; Palgrave, R.; Al-Jamal, K.T. Bioinspired polymerization of quercetin to produce a curcumin-loaded nanomedicine with potent cytotoxicity and cancer-targeting potential in vivo. ACS Biomater. Sci. Eng. 2019, 5, 6036–6045. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Orainee, B.; Alqudah, D.A.; Daoud, F.; Alshaer, W. Curcumin-Tannic Acid-Poloxamer Nanoassemblies Enhance Curcumin’s Uptake and Bioactivity against Cancer Cells In Vitro. Int. J. Pharm. 2021, 610, 121255. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Raghav, N. In-vitro studies of Curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Parmar, V.; Patel, G.; Abu-Thabit, N.Y. Responsive cyclodextrins as polymeric carriers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 555–580. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- National Center for Biotechnology. Information Beta-CYCLODEXTRIN. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-CYCLODEXTRIN (accessed on 19 October 2021).
- Obaidat, R.; Al-Shar’i, N.; Tashtoush, B.; Athamneh, T. Enhancement of levodopa stability when complexed with β-cyclodextrin in transdermal patches. Pharm. Dev. Technol. 2018, 23, 986–997. [Google Scholar] [CrossRef]
- Silberberg, M. Cyclodextrin as a Drug Carrier Increasing Drug Solubility. Sci. J. Lander Coll. Arts Sci. 2017, 11, 5. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, A.; Obaidat, R. Development and evaluation of fast-dissolving tablets of meloxicam-[beta]-cyclodextrin complex prepared by direct compression. Acta Pharm. 2011, 61, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Electrospun Polymer-Free Nanofibers Incorporating Hydroxypropyl-β-cyclodextrin/Difenoconazole via Supramolecular Assembly for Antifungal Activity. J. Agric. Food Chem. 2021, 69, 5871–5881. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloids Surf. B Biointerfaces 2021, 201, 111625. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Jiang, J.; Zhao, L.; Fu, Y.; Ye, F. Fabrication and characterization of thiophanate methyl/hydroxypropyl-β-cyclodextrin inclusion complex nanofibers by electrospinning. J. Mol. Liq. 2021, 335, 116228. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. An environmentally safe formulation with enhanced solubility and fungicidal activity: Self-assembly and characterization of Difenoconazole-β-CD inclusion complex. J. Mol. Liq. 2021, 327, 114874. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Fabrication of optimally controlled electrosprayed polymer-free nano-particles of curcumin/β-cyclodextrin inclusion complex. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126504. [Google Scholar] [CrossRef]
- Trotta, F. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine. 2011. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/book/10.1002/9780470926819 (accessed on 12 November 2021).
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanosponges: Synthesis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Trotta, F. Cyclodextrin nanosponges and their applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 323–342. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F.; Ferruti, P.; Ranucci, E.; Gerges, I.; Manfredi, A.; Marinotto, D.; Vavia, P. In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 183–191. [Google Scholar] [CrossRef]
- Dora, C.P.; Trotta, F.; Kushwah, V.; Devasari, N.; Singh, C.; Suresh, S.; Jain, S. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr. Polym. 2016, 137, 339–349. [Google Scholar] [CrossRef]
- Darandale, S.; Vavia, P. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Singireddy, A.; Subramanian, S. Cyclodextrin nanosponges to enhance the dissolution profile of quercetin by inclusion complex formation. Part. Sci. Technol. 2016, 34, 341–346. [Google Scholar] [CrossRef]
- Kamble, M.; Zaheer, Z.; Mokale, S.; Zainuddin, R. Formulation Optimization and Biopharmaceutical Evaluation of Imatinib Mesylate Loaded β-cyclodextrin Nanosponges. Pharm. Nanotechnol. 2019, 7, 343–361. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Bhingole, R.C. Nanosponge-based pediatric-controlled release dry suspension of Gabapentin for reconstitution. Drug Dev. Ind. Pharm. 2015, 41, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Falke, J.; Parkkinen, J.; Vaahtera, L.; Hulsbergen-van de Kaa, C.; Oosterwijk, E.; Witjes, J. Curcumin as treatment for bladder cancer: A preclinical study of cyclodextrin-curcumin complex and BCG as intravesical treatment in an orthotopic bladder cancer rat model. BioMed Res. Int. 2018, 2018, 9634902. [Google Scholar] [CrossRef]
- Gularte, M.S.; Quadrado, R.F.; Pedra, N.S.; Soares, M.S.; Bona, N.P.; Spanevello, R.M.; Fajardo, A.R. Preparation, characterization and antitumor activity of a cationic starch-derivative membrane embedded with a β-cyclodextrin/curcumin inclusion complex. Int. J. Biol. Macromol. 2020, 148, 140–152. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Mortezazadeh, T.; Salehian, F.; Ramazani, A.; Amanlou, M.; Khoobi, M. Improved curcumin loading, release, solubility and toxicity by tuning the molar ratio of cross-linker to β-cyclodextrin. Carbohydr. Polym. 2019, 213, 70–78. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Möller, K.; Macaulay, B.; Bein, T. Curcumin Encapsulated in Crosslinked Cyclodextrin Nanoparticles Enables Immediate Inhibition of Cell Growth and Efficient Killing of Cancer Cells. Nanomaterials 2021, 11, 489. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 1–21. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride cross-linked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Gharakhloo, M.; Sadjadi, S.; Rezaeetabar, M.; Askari, F.; Rahimi, A. Cyclodextrin-Based Nanosponges for Improving Solubility and Sustainable Release of Curcumin. ChemistrySelect 2020, 5, 1734–1738. [Google Scholar] [CrossRef]
- Guernelli, S.; Cariola, A.; Baschieri, A.; Amorati, R.; Meo, P.L. Nanosponges for the protection and release of the natural phenolic antioxidants quercetin, curcumin and phenethyl caffeate. Mater. Adv. 2020, 1, 2501–2508. [Google Scholar] [CrossRef]
- Asela, I.; Donoso-González, O.; Yutronic, N.; Sierpe, R. β-Cyclodextrin-Based Nanosponges Functionalized with Drugs and Gold Nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. A simple reversed phase high-performance liquid chromatography (HPLC) method for determination of in situ gelling curcumin-loaded liquid crystals in in vitro performance tests. Arab. J. Chem. 2017, 10, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Singh, R.; Tønnesen, H.H.; Vogensen, S.B.; Loftsson, T.; Másson, M. Studies of curcumin and curcuminoids. XXXVI. The stoichiometry and complexation constants of cyclodextrin complexes as determined by the phase-solubility method and UV–Vis titration. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 335–348. [Google Scholar] [CrossRef]
- Higuchi, T. A phase solubility technique. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Kfoury, M.; Landy, D.; Ruellan, S.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Determination of formation constants and structural characterization of cyclodextrin inclusion complexes with two phenolic isomers: Carvacrol and thymol. Beilstein J. Org. Chem. 2016, 12, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ja, M.H.; Kamal, N.; Hui, B.Y.; Fahmi, M. Inclusion of curcumin in β-cyclodextrins as potential drug delivery system: Preparation, characterization and its preliminary cytotoxicity approaches. Sains Malays. 2018, 47, 977–989. [Google Scholar]
- Jahed, V.; Zarrabi, A.; Bordbar, A.-k.; Hafezi, M.S. NMR (1H, ROESY) spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin and curcumin. Food Chem. 2014, 165, 241–246. [Google Scholar] [CrossRef]
- Loron, A.; Gardrat, C.; Tabary, N.; Martel, B.; Coma, V. Tetrahydrocurcumin encapsulation in cyclodextrins for water solubility improvement: Synthesis, characterization and antifungal activity as a new biofungicide. Carbohydr. Polym. Technol. Appl. 2021, 2, 100113. [Google Scholar] [CrossRef]
- Hegge, A.B.; Másson, M.; Kristensen, S.; Tønnesen, H.H. Investigation of curcumin-cyclodextrin inclusion complexation in aqueous solutions containing various alcoholic co-solvents and alginates using an UV-VIS titration method. Die Pharm.-Int. J. Pharm. Sci. 2009, 64, 382–389. [Google Scholar]
- Patro, N.M.; Sultana, A.; Terao, K.; Nakata, D.; Jo, A.; Urano, A.; Ishida, Y.; Gorantla, R.N.; Pandit, V.; Devi, K. Comparison and correlation of in vitro, in vivo and in silico evaluations of alpha, beta and gamma cyclodextrin complexes of curcumin. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 471–483. [Google Scholar] [CrossRef]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J. Nanopart. Res. 2008, 10, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Nasra, M.M.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Connors, K.A. Population characteristics of cyclodextrin complex stabilities in aqueous solution. J. Pharm. Sci. 1995, 84, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of curcumin/cyclodextrin polymer inclusion complex and investigation on its antioxidant and antiproliferative activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.; Parveen, R. Solubility and stability enhancement of curcumin: Improving drug properties of natural pigment. Drug Dev. Ther. 2016, 7, 113–117. [Google Scholar] [CrossRef]
- Yaşayan, G.; Şatıroğlu Sert, B.; Tatar, E.; Küçükgüzel, İ. Fabrication and characterisation studies of cyclodextrin-based nanosponges for sulfamethoxazole delivery. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 175–186. [Google Scholar] [CrossRef]
- Castiglione, F.; Crupi, V.; Majolino, D.; Mele, A.; Rossi, B.; Trotta, F.; Venuti, V. Inside new materials: An experimental numerical approach for the structural elucidation of nanoporous cross-linked polymers. J. Phys. Chem. B 2012, 116, 13133–13140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, B.; Caponi, S.; Castiglione, F.; Corezzi, S.; Fontana, A.; Giarola, M.; Mariotto, G.; Mele, A.; Petrillo, C.; Trotta, F. Networking properties of cyclodextrin-based cross-linked polymers probed by inelastic light-scattering experiments. J. Phys. Chem. B 2012, 116, 5323–5327. [Google Scholar] [CrossRef]
- Castiglione, F.; Crupi, V.; Majolino, D.; Mele, A.; Rossi, B.; Trotta, F.; Venuti, V. Effect of cross-linking properties on the vibrational dynamics of cyclodextrins-based polymers: An experimental–numerical study. J. Phys. Chem. B 2012, 116, 7952–7958. [Google Scholar] [CrossRef]
- Crupi, V.; Fontana, A.; Giarola, M.; Majolino, D.; Mariotto, G.; Mele, A.; Melone, L.; Punta, C.; Rossi, B.; Trotta, F. Connection between the vibrational dynamics and the cross-linking properties in cyclodextrins-based polymers. J. Raman Spectrosc. 2013, 44, 1457–1462. [Google Scholar] [CrossRef]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. DARU J. Pharm. Sci. 2020, 28, 1–18. [Google Scholar]
- Al-Shar’i, N.A.; Obaidat, R.M. Experimental and computational comparative study of the supercritical fluid technology (SFT) and kneading method in preparing β-cyclodextrin complexes with two essential oils (Linalool and Carvacrol). AAPS Pharm. Sci. Tech. 2018, 19, 1037–1047. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular inclusion complex of curcumin-β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Wani, K.; Kaul-Ghanekar, R.; Prabhune, A.; Ogale, S. From micron to nano-curcumin by sophorolipid co-processing: Highly enhanced bioavailability, fluorescence, and anti-cancer efficacy. RSC Adv. 2014, 4, 60334–60341. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabih Maria, D.; R Mishra, S.; Wang, L.; Helmy Abd-Elgawad, A.-E.; Abd-Elazeem Soliman, O.; Salah El-Dahan, M.; M Jablonski, M. Water-soluble complex of curcumin with cyclodextrins: Enhanced physical properties for ocular drug delivery. Curr. Drug Deliv. 2017, 14, 875–886. [Google Scholar]
- Kasapoglu-Calik, M.; Ozdemir, M. Synthesis and controlled release of curcumin-β-cyclodextrin inclusion complex from nanocomposite poly (N-isopropylacrylamide/sodium alginate) hydrogels. J. Appl. Polym. Sci. 2019, 136, 47554. [Google Scholar] [CrossRef]
- Ismail, E.; Sabry, D.; Mahdy, H.; Khalil, M. Synthesis and Characterization of some Ternary Metal Complexes of Curcumin with 1, 10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Radjaram, A.; Hafid, A.F.; Setyawan, D. Dissolution enhancement of curcumin by hydroxypropyl-β-cyclodextrin complexation. Int. J. Pharm. Pharm. Sci. 2013, 5, 401–405. [Google Scholar]
- Rezaei, A.; Nasirpour, A. Evaluation of release kinetics and mechanisms of curcumin and curcumin-β-cyclodextrin inclusion complex incorporated in electrospun almond gum/PVA nanofibers in simulated saliva and simulated gastrointestinal conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Anaya-Castro, M.A.; Ayala-Zavala, J.F.; Muñoz-Castellanos, L.; Hernández-Ochoa, L.; Peydecastaing, J.; Durrieu, V. β-Cyclodextrin inclusion complexes containing clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils: Preparation, physicochemical and antimicrobial characterization. Food Packag. Shelf Life 2017, 14, 96–101. [Google Scholar] [CrossRef] [Green Version]








| Equation | R2 | Kc (M−1) | Complexation Efficiency | Ratio 1 | Type of Curve | |
|---|---|---|---|---|---|---|
| β-Cyclodextrin | Y = 0.0258X + 0.0561 | 0.994 | 487.34 | 0.03 | 1:1 | AN |
| NS4 | Y = 0.2634X + 0.0477 | 0.994 | 4972.90 | 0.26 | 1:1 | BS |
| NS6 | Y = 0.2206X + 0.0522 | 0.998 | 4164.50 | 0.22 | 1:1 | BS |
| NS8 | Y = 0.1891X + 0.0512 | 0.997 | 3567.87 | 0.19 | 1:1 | BS |
| Zeta Potential | SD | Size (nm) | SD | PDI | SD | |
|---|---|---|---|---|---|---|
| β-Cyclodextrin Complex 1 | 14.6 ± 2.33 (64.8%) −25.9 ± 3.53 (35.3%) | 2.25 | 6759 | 1762.11 | 0.201 | 0.015 |
| NS4 | −21.57 | 4.85 | 266.6 | 15.84 | 0.321 | 0.044 |
| Raw β-Cyclodextrin | −29.10 | 5.37 | 3514 | 2554.07 | 0.533 | 0.455 |
| Plain NS4 | −18.30 | 4.67 | 85.74 | 1.99 | 0.265 | 0.021 |
| Raw Curcumin | −24.2 | 2.25 | 5956.5 | 968.03 | 0.493 | 0.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234073
Mashaqbeh H, Obaidat R, Al-Shar’i N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers. 2021; 13(23):4073. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234073
Chicago/Turabian StyleMashaqbeh, Hadeia, Rana Obaidat, and Nizar Al-Shar’i. 2021. "Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation" Polymers 13, no. 23: 4073. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234073