A Simple Approach to Control the Physical and Chemical Features of Custom-Synthesized N-Doped Carbon Nanotubes and the Extent of Their Network Formation in Polymers: The Importance of Catalyst to Substrate Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.2.1. Catalyst and N-MWNT Characterization
2.2.2. Nanocomposites Morphology and Structure
3. Results and Discussion
3.1. Characterization of N-MWNTs
3.1.1. Transmission Electron Microscopy
3.1.2. X-ray Photoelectron Spectroscopy
3.1.3. Raman Spectroscopy
3.1.4. Thermogravimetric Analysis (TGA)
3.2. Characterization of N-MWNTs/PVDF Nanocomposites
3.2.1. Morphology of N-MWNTs/PVDF Nanocomposites
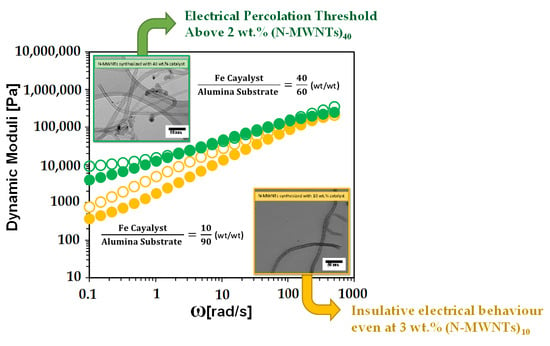
3.2.2. Rheology of N-MWNTs/PVDF Nanocomposites
3.2.3. Lissajous-Bowditch Plots
3.2.4. Broadband Electrical Conductivity of N-MWNTs/PVDF Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Kong, H.; Gao, C.; Yan, D. Functionalization of multiwalled carbon nanotubes by atom transfer radical polymerization and defunctionalization of the products. Macromolecules 2004, 37, 4022–4030. [Google Scholar] [CrossRef]
- Bellucci, S. Carbon nanotubes: Physics and applications. Phys. Status Solidi C 2005, 2, 34–47. [Google Scholar] [CrossRef]
- Kamkar, M.; Sadeghi, S.; Arjmand, M.; Sundararaj, U. Structural characterization of CVD custom-synthesized carbon nanotube/polymer nanocomposites in large-amplitude oscillatory shear (LAOS) mode: Effect of dispersion characteristics in confined geometries. Macromolecules 2019, 52, 1489–1504. [Google Scholar] [CrossRef]
- Su, M.; Zheng, B.; Liu, J. A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst productivity. Chem. Phys. Lett. 2000, 322, 321–326. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Ayatollahi, M.; Shadlou, S.; Shokrieh, M.; Chitsazzadeh, M. Effect of multi-walled carbon nanotube aspect ratio on mechanical and electrical properties of epoxy-based nanocomposites. Polym. Test. 2011, 30, 548–556. [Google Scholar] [CrossRef]
- Erfanian, E.; Kamkar, M.; Williams, D.; Zamani Keteklahijani, Y.; Salehiyan, R.; Ray, S.S.; Arjmand, M.; Sundararaj, U. Dielectrorheology of Aspect-Ratio-Tailored Carbon Nanotube/Polyethylene Composites under Large Deformations: Implications for High-Temperature Dielectrics. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Arjmand, M.; Chizari, K.; Krause, B.; Pötschke, P.; Sundararaj, U. Effect of synthesis catalyst on structure of nitrogen-doped carbon nanotubes and electrical conductivity and electromagnetic interference shielding of their polymeric nanocomposites. Carbon 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, M.Y.; Li, J.; Shi, X.M.; Kim, J.K. Effects of surfactant treatment on mechanical and electrical properties of CNT/epoxy nanocomposites. Compos. Part A: Appl. Sci. Manuf. 2008, 39, 1876–1883. [Google Scholar] [CrossRef]
- Park, O.-K.; Jeevananda, T.; Kim, N.H.; Kim, S.-i.; Lee, J.H. Effects of surface modification on the dispersion and electrical conductivity of carbon nanotube/polyaniline composites. Scr. Mater. 2009, 60, 551–554. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Kamkar, M.; Riazi, H.; Hosseini, E.; Dijvejin, Z.A.; Golovin, K.; Soroush, M.; Arjmand, M. Scalable Manufacturing of Flexible and Highly Conductive Ti3C2/PEDOT: PSS Thin Films for Electromagnetic Interference Shielding. New J. Chem. 2021, 45, 20787–20799. [Google Scholar] [CrossRef]
- Kamkar, M.; Ghaffarkhah, A.; Hosseini, E.; Amini, M.; Ghaderi, S.; Arjmand, M. Multilayer Polymeric Nanocomposites for Electromagnetic Interference Shielding: Fabrication, Mechanisms, and Prospects. New J. Chem. 2021. [Google Scholar] [CrossRef]
- Yang, C.; Lin, Y.; Nan, C. Modified carbon nanotube composites with high dielectric constant, low dielectric loss and large energy density. Carbon 2009, 47, 1096–1101. [Google Scholar] [CrossRef]
- Liu, H.; Shen, Y.; Song, Y.; Nan, C.W.; Lin, Y.; Yang, X. Carbon nanotube array/polymer core/shell structured composites with high dielectric permittivity, low dielectric loss, and large energy density. Adv. Mater. 2011, 23, 5104–5108. [Google Scholar] [CrossRef]
- Arjmand, M.; Sundararaj, U. Impact of BaTiO3 as insulative ferroelectric barrier on the broadband dielectric properties of MWCNT/PVDF nanocomposites. Polym. Compos. 2016, 37, 299–304. [Google Scholar] [CrossRef]
- Arjmand, M.; Sadeghi, S.; Khajehpour, M.; Sundararaj, U. Carbon nanotube/graphene nanoribbon/polyvinylidene fluoride hybrid nanocomposites: Rheological and dielectric properties. J. Phys. Chem. C 2017, 121, 169–181. [Google Scholar] [CrossRef]
- Keteklahijani, Y.Z.; Arjmand, M.; Sundararaj, U. Cobalt catalyst grown carbon nanotube/poly (vinylidene fluoride) nanocomposites: Effect of synthesis temperature on morphology, electrical conductivity and electromagnetic interference shielding. ChemistrySelect 2017, 2, 10271–10284. [Google Scholar] [CrossRef]
- Chen, G. Synthesis of carbon nanostructures on nanocrystalline Ni-Ni3P catalyst supported by SiC whiskers. Carbon 2003, 41, 579. [Google Scholar]
- Arjmand, M.; Sundararaj, U. Effects of nitrogen doping on X-band dielectric properties of carbon nanotube/polymer nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 17844–17850. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, K.; Yang, S.; Naito, H.; Kotani, M.; Sato, S.; Isobe, H. A nitrogen-doped nanotube molecule with atom vacancy defects. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Wang, N.; Wu, Z.; Li, L. Probing active sites on metal-free, nitrogen-doped carbons for oxygen electroreduction: A review. Catalysts 2018, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lee, D.-M.; Park, M.; Wee, J.-H.; Jeong, H.S.; Ku, B.-C.; Yang, C.-M.; Lee, D.S.; Terrones, M.; Kim, Y.A. Controlled synthesis of N-type single-walled carbon nanotubes with 100% of quaternary nitrogen. Carbon 2020, 167, 881–887. [Google Scholar] [CrossRef]
- Ghosh, K.; Kumar, M.; Maruyama, T.; Ando, Y. Controllable growth of highly N-doped carbon nanotubes from imidazole: A structural, spectroscopic and field emission study. J. Mater. Chem. 2010, 20, 4128–4134. [Google Scholar] [CrossRef]
- Glerup, M.; Castignolles, M.; Holzinger, M.; Hug, G.; Loiseau, A.; Bernier, P. Synthesis of highly nitrogen-doped multi-walled carbon nanotubes. Chem. Commun. 2003, 2542–2543. [Google Scholar] [CrossRef]
- Kamkar, M.; Sadeghi, S.; Arjmand, M.; Aliabadian, E.; Sundararaj, U. Intra-cycle elastic nonlinearity of nitrogen-doped carbon nanotube/polymer nanocomposites under medium amplitude oscillatory shear (MAOS) flow. Nanomaterials 2020, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; Morin, S.; Stevenson, K.J. Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 2006, 44, 1429–1437. [Google Scholar] [CrossRef]
- Villalpando-Paez, F.; Zamudio, A.; Elias, A.; Son, H.; Barros, E.; Chou, S.; Kim, Y.; Muramatsu, H.; Hayashi, T.; Kong, J. Synthesis and characterization of long strands of nitrogen-doped single-walled carbon nanotubes. Chem. Phys. Lett. 2006, 424, 345–352. [Google Scholar] [CrossRef]
- Barzegar, H.R.; Gracia-Espino, E.; Sharifi, T.; Nitze, F.; Wågberg, T. Nitrogen doping mechanism in small diameter single-walled carbon nanotubes: Impact on electronic properties and growth selectivity. J. Phys. Chem. C 2013, 117, 25805–25816. [Google Scholar] [CrossRef]
- Chizari, K.; Vena, A.; Laurentius, L.; Sundararaj, U. The effect of temperature on the morphology and chemical surface properties of nitrogen-doped carbon nanotubes. Carbon 2014, 68, 369–379. [Google Scholar] [CrossRef]
- Jadhav, P.; Joshi, G.M. Recent trends in Nitrogen doped polymer composites: A review. J. Polym. Res. 2021, 28, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.-S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507. [Google Scholar] [CrossRef]
- Pawar, S.P.; Arjmand, M.; Pötschke, P.; Krause, B.; Fischer, D.; Bose, S.; Sundararaj, U. Tuneable dielectric properties derived from nitrogen-doped carbon nanotubes in PVDF-based nanocomposites. ACS Omega 2018, 3, 9966–9980. [Google Scholar] [CrossRef]
- Pawar, S.P.; Arjmand, M.; Gandi, M.; Bose, S.; Sundararaj, U. Critical insights into understanding the effects of synthesis temperature and nitrogen doping towards charge storage capability and microwave shielding in nitrogen-doped carbon nanotube/polymer nanocomposites. RSC Adv. 2016, 6, 63224–63234. [Google Scholar] [CrossRef]
- Sharifi, T.; Nitze, F.; Barzegar, H.R.; Tai, C.-W.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wågberg, T. Nitrogen doped multi walled carbon nanotubes produced by CVD-correlating XPS and Raman spectroscopy for the study of nitrogen inclusion. Carbon 2012, 50, 3535–3541. [Google Scholar] [CrossRef]
- Czerw, R.; Terrones, M.; Charlier, J.-C.; Blase, X.; Foley, B.; Kamalakaran, R.; Grobert, N.; Terrones, H.; Tekleab, D.; Ajayan, P. Identification of electron donor states in N-doped carbon nanotubes. Nano Lett. 2001, 1, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Arenal, R.; March, K.; Ewels, C.P.; Rocquefelte, X.; Kociak, M.; Loiseau, A.; Stéphan, O. Atomic configuration of nitrogen-doped single-walled carbon nanotubes. Nano Lett. 2014, 14, 5509–5516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, N.; Ma, Y.; Du, F.; Li, F.; He, X.; Lin, X.; Gao, H.; Chen, Y. The influence of single-walled carbon nanotube structure on the electromagnetic interference shielding efficiency of its epoxy composites. Carbon 2007, 45, 1614–1621. [Google Scholar] [CrossRef]
- Singh, B.; Saini, K.; Choudhary, V.; Teotia, S.; Pande, S.; Saini, P.; Mathur, R. Effect of length of carbon nanotubes on electromagnetic interference shielding and mechanical properties of their reinforced epoxy composites. J. Nanoparticle Res. 2014, 16, 2161. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Li, H.; Gao, J.; Zhang, J.; Zeng, Y. Effect of carbon nanotubes shape on the properties of multiwall carbon nanotubes/polyethylene flexible transparent conductive films. J. Mater. Sci. Mater. Electron. 2014, 25, 2692–2696. [Google Scholar] [CrossRef]
- Van Dommele, S.; de Jong, K.P.; Romero-Izquirdo, A.; Bitter, J. Synthesis of heterogeneous base catalysts: Nitrogen containing carbon nanotubes. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2006; Volume 162, pp. 29–36. [Google Scholar]
- Pels, J.; Kapteijn, F.; Moulijn, J.; Zhu, Q.; Thomas, K. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Van Dommele, S.; Romero-Izquirdo, A.; Brydson, R.; De Jong, K.; Bitter, J. Tuning nitrogen functionalities in catalytically grown nitrogen-containing carbon nanotubes. Carbon 2008, 46, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Faba, L.; Criado, Y.A.; Gallegos-Suárez, E.; Pérez-Cadenas, M.; Díaz, E.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Ordóñez, S. Preparation of nitrogen-containing carbon nanotubes and study of their performance as basic catalysts. Appl. Catal. A: Gen. 2013, 458, 155–161. [Google Scholar] [CrossRef]
- Muñoz-Sandoval, E.; Fajardo-Díaz, J.L.; Sánchez-Salas, R.; Cortés-López, A.J.; López-Urías, F. Two sprayer CVD synthesis of nitrogen-doped carbon sponge-type nanomaterials. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- Ameli, A.; Arjmand, M.; Pötschke, P.; Krause, B.; Sundararaj, U. Effects of synthesis catalyst and temperature on broadband dielectric properties of nitrogen-doped carbon nanotube/polyvinylidene fluoride nanocomposites. Carbon 2016, 106, 260–278. [Google Scholar] [CrossRef]
- Boncel, S.; Pattinson, S.W.; Geiser, V.; Shaffer, M.S.; Koziol, K.K. En route to controlled catalytic CVD synthesis of densely packed and vertically aligned nitrogen-doped carbon nanotube arrays. Beilstein J. Nanotechnol. 2014, 5, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Kamkar, M.; Aliabadian, E.; Shayesteh Zeraati, A.; Sundararaj, U. Application of nonlinear rheology to assess the effect of secondary nanofiller on network structure of hybrid polymer nanocomposites. Phys. Fluids 2018, 30, 023102. [Google Scholar] [CrossRef]
- Sarvestani, A.S.; Jabbari, E. Modeling the viscoelastic response of suspension of particles in polymer solution: The effect of polymer-particle interactions. Macromol. Theory Simul. 2007, 16, 378–385. [Google Scholar] [CrossRef]
- Hsiao, L.C.; Newman, R.S.; Glotzer, S.C.; Solomon, M.J. Role of isostaticity and load-bearing microstructure in the elasticity of yielded colloidal gels. Proc. Natl. Acad. Sci. USA 2012, 109, 16029–16034. [Google Scholar] [CrossRef] [Green Version]
- Kamkar, M.; Sultana, S.N.; Pawar, S.P.; Eshraghian, A.; Erfanian, E.; Sundararaj, U. The key role of processing in tuning nonlinear viscoelastic properties and microwave absorption in CNT-based polymer nanocomposites. Mater. Today Commun. 2020, 24, 101010. [Google Scholar] [CrossRef]
- Otero Navas, I.; Kamkar, M.; Arjmand, M.; Sundararaj, U. Morphology Evolution, Molecular Simulation, Electrical Properties, and Rheology of Carbon Nanotube/Polypropylene/Polystyrene Blend Nanocomposites: Effect of Molecular Interaction between Styrene-Butadiene Block Copolymer and Carbon Nanotube. Polymers 2021, 13, 230. [Google Scholar] [CrossRef]
- Eshraghian, A.; Kamkar, M.; Asgari, M.; Arjmand, M.; Sundararaj, U. X-band dielectric properties of hybrid nanocomposites of nitrogen-doped carbon nanotube/functionalized nanoclay/polyvinylidene fluoride nanocomposite. Polym. Compos. 2021, 42, 1034–1048. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 609, 125577. [Google Scholar] [CrossRef]
- Ahmadian Hoseini, A.H.; Erfanian, E.; Kamkar, M.; Sundararaj, U.; Liu, J.; Arjmand, M. Waste to Value-Added Product: Developing Electrically Conductive Nanocomposites Using a Non-Recyclable Plastic Waste Containing Vulcanized Rubber. Polymers 2021, 13, 2427. [Google Scholar] [CrossRef]
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Nonlinear viscoelastic characterization of charged cellulose nanocrystal network structure in the presence of salt in aqueous media. Cellulose 2020, 27, 5729–5743. [Google Scholar] [CrossRef]
- Salehiyan, R.; Yoo, Y.; Choi, W.J.; Hyun, K. Characterization of morphologies of compatibilized polypropylene/polystyrene blends with nanoparticles via nonlinear rheological properties from FT-rheology. Macromolecules 2014, 47, 4066–4076. [Google Scholar] [CrossRef]
- Salehiyan, R.; Song, H.Y.; Choi, W.J.; Hyun, K. Characterization of effects of silica nanoparticles on (80/20) PP/PS blends via nonlinear rheological properties from Fourier transform rheology. Macromolecules 2015, 48, 4669–4679. [Google Scholar] [CrossRef]
- Salehiyan, R.; Song, H.Y.; Kim, M.; Choi, W.J.; Hyun, K. Morphological evaluation of PP/PS blends filled with different types of clays by nonlinear rheological analysis. Macromolecules 2016, 49, 3148–3160. [Google Scholar] [CrossRef]
- Song, H.Y.; Nnyigide, O.S.; Salehiyan, R.; Hyun, K. Investigation of nonlinear rheological behavior of linear and 3-arm star 1, 4-cis-polyisoprene (PI) under medium amplitude oscillatory shear (MAOS) flow via FT-rheology. Polymer 2016, 104, 268–278. [Google Scholar] [CrossRef]
- Sugimoto, M.; Suzuki, Y.; Hyun, K.; Ahn, K.H.; Ushioda, T.; Nishioka, A.; Taniguchi, T.; Koyama, K. Melt rheology of long-chain-branched polypropylenes. Rheol. Acta 2006, 46, 33–44. [Google Scholar] [CrossRef]
- Hyun, K.; Nam, J.G.; Wilhelm, M.; Ahn, K.H.; Lee, S.J. Nonlinear response of complex fluids under LAOS (large amplitude oscillatory shear) flow. Korea-Aust. Rheol. J. 2003, 15, 97–105. [Google Scholar]












| Sample Type | Component Assignment | Peak BE (eV) | Atomic Conc. (%) |
|---|---|---|---|
| (N-CNTs)10 | sp1-C | 283.67 | 3.76 |
| sp2-C | 284.67 | 81.11 | |
| sp3-C | 286.23 | 1.85 | |
| CO Bondings | 291.05 289.22 | 10.13 | |
| (N-CNTs)20 | sp1-C | 283.67 | 3.23 |
| sp2-C | 284.67 | 83.26 | |
| sp3-C | 286.23 | 5.94 | |
| CO Bondings | 291.05 289.22 | 7.75 | |
| (N-CNTs)40 | sp1-C | 283.67 | 3.37 |
| sp2-C | 284.67 | 89.01 | |
| sp3-C | 286.23 | 0.55 | |
| CO Bondings * | 291.05 289.22 | 7.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erfanian, E.; Kamkar, M.; Pawar, S.P.; Keteklahijani, Y.Z.; Arjmand, M.; Sundararaj, U. A Simple Approach to Control the Physical and Chemical Features of Custom-Synthesized N-Doped Carbon Nanotubes and the Extent of Their Network Formation in Polymers: The Importance of Catalyst to Substrate Ratio. Polymers 2021, 13, 4156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234156
Erfanian E, Kamkar M, Pawar SP, Keteklahijani YZ, Arjmand M, Sundararaj U. A Simple Approach to Control the Physical and Chemical Features of Custom-Synthesized N-Doped Carbon Nanotubes and the Extent of Their Network Formation in Polymers: The Importance of Catalyst to Substrate Ratio. Polymers. 2021; 13(23):4156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234156
Chicago/Turabian StyleErfanian, Elnaz, Milad Kamkar, Shital Patangrao Pawar, Yalda Zamani Keteklahijani, Mohammad Arjmand, and Uttandaraman Sundararaj. 2021. "A Simple Approach to Control the Physical and Chemical Features of Custom-Synthesized N-Doped Carbon Nanotubes and the Extent of Their Network Formation in Polymers: The Importance of Catalyst to Substrate Ratio" Polymers 13, no. 23: 4156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234156