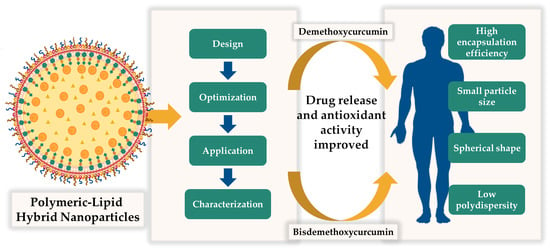
Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design and Optimization of the Polymer-Lipid Hybrid Nanoparticles (PLHNs) Method Using CUR as Model
2.2.1. Homogenization Technique
2.2.2. Lipid, Drug Loading, Lipid-Drug Ratio, and Organic Solvent
2.2.3. Surfactant Selection and Polymer Concentration
2.2.4. Preparation, Characterization, and In Vitro Evaluation of DMC and BDM-Loaded PLHNs
2.3. Characterization Techniques
2.3.1. Encapsulation Efficiency (EE)
2.3.2. Fourier Transform Infrared (FT-IR)
2.3.3. Dynamic Light Scattering (DLS)
2.3.4. High Resolution Transmission Electron Microscopy (HR-TEM)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. In Vitro Studies
Drug Release Profile
DPPH Radical-Scavenging Activity
3. Results
3.1. Method Design and Optimization Using CUR as Model
3.1.1. Homogenization Technique
3.1.2. Lipid, Drug Loading, Lipid-Drug Ratio, and Organic Solvent
3.1.3. Surfactant Selection and Polymer Concentration
3.2. Preparation, Characterization and In Vitro Evaluation of DMC and BDM-Loaded PLHNs
3.2.1. Encapsulation, FT-IR and EE
3.2.2. DLS and HR-TEM Techniques
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. In Vitro Studies
Drug Release
Antioxidant Activity Evaluation of DMC and BDMC Free and Loaded into PLHNs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Madkour, L.H. Nanoparticle and polymeric nanoparticle-based targeted drug delivery systems. In Nucleic Acids as Gene Anticancer Drug Delivery Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–240. [Google Scholar]
- Cheow, W.S.; Hadinoto, K. Enhancing encapsulation efficiency of highly water-soluble antibiotic in poly(lactic-co-glycolic acid) nanoparticles: Modifications of standard nanoparticle preparation methods. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 79–86. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Ahmed, N.; .ur. Rehman, A.; Khan, G.M. Lipid polymer hybrid carrier systems for cancer targeting: A review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 86–100. [Google Scholar] [CrossRef]
- Jose, C.; Amra, K.; Bhavsar, C.; Momin, M.; Omri, A. Polymeric Lipid Hybrid Nanoparticles: Properties and Therapeutic Applications. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 555–588. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Li, L.; Dong, H.-Q.; Cai, X.-J.; Ren, T.-B. Pluronic F127 nanomicelles engineered with nuclear localized functionality for targeted drug delivery. Mater. Sci. Eng. C 2013, 33, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic? Block Copolymers in Drug Delivery: From Micellar Nanocontainers to Biological Response Modifiers. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.S.S.; Marín Huachaca, N.S.; Lemos, M.; Santos, N.F.; Feitosa, E.; Salay, L.C. Molecular interactions between Pluronic F127 and saponin in aqueous solution. Colloid Polym. Sci. 2020, 298, 113–122. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of Curcumin in Pluronic Block Copolymer Micelles for Drug Delivery Applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Kumar, S.; Kunwar, A.; Nath, S.; Sarma, H.D.; Tripathi, A.; Verma, G.; Chaudhari, D.P.; Aswal, V.K.; Melo, J.S. Structural and therapeutic properties of curcumin solubilized pluronic F127 micellar solutions and hydrogels. J. Mol. Liq. 2020, 314, 113591. [Google Scholar] [CrossRef]
- Enumo, A.; Argenta, D.F.; Bazzo, G.C.; Caon, T.; Stulzer, H.K.; Parize, A.L. Development of curcumin-loaded chitosan/pluronic membranes for wound healing applications. Int. J. Biol. Macromol. 2020, 163, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Cho, D.-C.; Kim, C.H.; Han, I.; Gil, E.Y.; Kim, K.-T. Effect of curcumin on the inflammatory reaction and functional recovery after spinal cord injury in a hyperglycemic rat model. Spine J. 2019, 19, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Heffernan, C.; Ukrainczyk, M.; Gamidi, R.K.; Hodnett, B.K.; Rasmuson, Å.C. Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Org. Process Res. Dev. 2017, 21, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Ukrainczyk, M.; Hodnett, B.K.; Rasmuson, Å.C. Process Parameters in the Purification of Curcumin by Cooling Crystallization. Org. Process Res. Dev. 2016, 20, 1593–1602. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of curcuminoids and evaluation of their cytotoxic and antioxidant properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Sahebkar, A. Demethoxycurcumin: A naturally occurring curcumin analogue for treating non-cancerous diseases. J. Cell. Physiol. 2019, 234, 19320–19330. [Google Scholar] [CrossRef] [PubMed]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Si, X.; Han, M.K.; Viennois, E.; Zhang, M.; Merlin, D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B 2015, 3, 7724–7733. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-D.; Kim, J.A.; Kwak, M.K.; Yoo, B.K.; Yong, C.S.; Choi, H.-G. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol. Pharm. Bull. 2011, 34, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Yen, F.-L.; Wu, T.-H.; Tzeng, C.-W.; Lin, L.-T.; Lin, C.-C. Curcumin Nanoparticles Improve the Physicochemical Properties of Curcumin and Effectively Enhance Its Antioxidant and Antihepatoma Activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.-M.; Faivre, J.; Tehrani, S.F.; Lalloz, A.; Hildgen, P.; Banquy, X. Effect of the Polymer Architecture on the Structural and Biophysical Properties of PEG–PLA Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 10374–10385. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Natarajan, S.R.; Thangaraj, K.; Manoharan, R. Therapeutic aspects of AMPK in breast cancer: Progress, challenges, and future directions. Biochim. Biophys. Acta-Rev. Cancer 2020, 1874, 188379. [Google Scholar] [CrossRef]
- Guo, L.Y.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Shin, E.M.; Zhou, H.Y.; Jung, J.W.; Kim, Y.S. Comparison of suppressive effects of demethoxycurcumin and bisdemethoxycurcumin on expressions of inflammatory mediators In Vitro and In Vivo. Arch. Pharm. Res. 2008, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Schiborr, C.; Behnam, D.; Frank, J. The oral bioavailability of curcuminoids in healthy humans is markedly enhanced by micellar solubilisation but not further improved by simultaneous ingestion of sesamin, ferulic acid, naringenin and xanthohumol. J. Funct. Foods 2015, 14, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Sudeep, V.H.; Gouthamchandra, K.; Chandrappa, S.; Naveen, P.; Reethi, B.; Venkatakrishna, K.; Shyamprasad, K. In vitro gastrointestinal digestion of a bisdemethoxycurcumin-rich Curcuma longa extract and its oral bioavailability in rats. Bull. Natl. Res. Cent. 2021, 45, 84. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18. [Google Scholar] [CrossRef]
- Udompornmongkol, P.; Chiang, B.-H. Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J. Biomater. Appl. 2015, 30, 537–546. [Google Scholar] [CrossRef]
- Godara, S.; Lather, V.; Kirthanashri, S.V.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2020, 109, 110576. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Arnaez, E.; Moreira, I.; Hurtado, A.; Monge, D.; Monagas, M. Polyphenolic composition and antioxidant activity of Uncaria tomentosa commercial bark products. Antioxidants 2019, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules 2021, 26, 6493. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L. Lipid–Polymer Hybrid Nanoparticles: Synthesis, Characterization and Applications. Nano LIFE 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Malekpour, M.; Naghibzadeh, M.; Mohammad, R.; Esnaashari, S.S.; Adabi, M.; Mujokoro, B.; Khosravani, M.; Adabi, M. Effect of various parameters on encapsulation efficiency of mPEG-PLGA nanoparticles: Artificial neural network. Biointerface Res. Appl. Chem. 2018, 8, 3267–3272. [Google Scholar]
- Aben, S.; Holtze, C.; Tadros, T.; Schurtenberger, P. Rheological Investigations on the Creaming of Depletion-Flocculated Emulsions. Langmuir 2012, 28, 7967–7975. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef]
- Sezgin, Z.; Yuksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sharma, R.; Shaikh, S.; Ray, D.; Aswal, V.K.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2019, 2, e1133. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, K.; Jose, J. Development of amphotericin b Based organogels against mucocutaneous fungal infections. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.; Kumar, V.; Khajuria, Y. Spectroscopic Studies of Cholesterol: Fourier Transform Infra-Red and Vibrational Frequency Analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Hajj Ali, H.; Michaux, F.; Bouelet Ntsama, I.S.; Durand, P.; Jasniewski, J.; Linder, M. Shea butter solid nanoparticles for curcumin encapsulation: Influence of nanoparticles size on drug loading. Eur. J. Lipid Sci. Technol. 2016, 118, 1168–1178. [Google Scholar] [CrossRef]
- Valencia, M.S.; da Silva Júnior, M.F.; Xavier-Júnior, F.H.; Veras, B.d.O.; Albuquerque, P.B.S.d.; Borba, E.F.d.O.; Silva, T.G.d.; Xavier, V.L.; Souza, M.P.d.; Carneiro-da-Cunha, M.d.G. Characterization of curcumin-loaded lecithin-chitosan bioactive nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100119. [Google Scholar] [CrossRef]
- Saedi, A.; Rostamizadeh, K.; Parsa, M.; Dalali, N.; Ahmadi, N. Preparation and characterization of nanostructured lipid carriers as drug delivery system: Influence of liquid lipid types on loading and cytotoxicity. Chem. Phys. Lipids 2018, 216, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-Loaded Lecithin/Chitosan Nanoparticles for Functional Food Applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Wang, Q.; Liu, J.; Liu, J.; Thant, Y.; Weng, W.; Wei, C.; Bao, R.; Adu-Frimpong, M.; Yu, Q.; Deng, W.; et al. Bisdemethoxycurcumin-conjugated vitamin E TPGS liposomes ameliorate poor bioavailability of free form and evaluation of its analgesic and hypouricemic activity in oxonate-treated rats. J. Nanoparticle Res. 2021, 23, 122. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Karimi, M.; Nasirizadeh, S.; Hatamipour, M.; Golmohammadzadeh, S.; Jaafari, M.R. Preparation, characterization and in vivo pharmacokinetic evaluation of curcuminoids-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). J. Drug Deliv. Sci. Technol. 2021, 62, 102352. [Google Scholar] [CrossRef]
- Ahmad, N.; Umar, S.; Ashafaq, M.; Akhtar, M.; Iqbal, Z.; Samim, M.; Ahmad, F.J. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma 2013, 250, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.; Omari-Siaw, E.; Adu-Frimpong, M.; Liu, J.; Xu, X.; Yu, J. Enhanced oral bioavailability of Bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: Formulation design, in vitro and in vivo evaluation. Int. J. Pharm. 2020, 590, 119887. [Google Scholar] [CrossRef]
- Van Dooren, A.A. Design for Drug-Excipient Interaction Studies. Drug Dev. Ind. Pharm. 1983, 9, 43–55. [Google Scholar] [CrossRef]
- Lütfi, G.; Müzeyyen, D. Preparation and characterization of polymeric and lipid nanoparticles of pilocarpine HCl for ocular application. Pharm. Dev. Technol. 2013, 18, 701–709. [Google Scholar] [CrossRef]
- Péret-Almeida, L.; Cherubino, A.P.F.; Alves, R.J.; Dufossé, L.; Glória, M.B.A. Separation and determination of the physico-chemical characteristics of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Res. Int. 2005, 38, 1039–1044. [Google Scholar] [CrossRef]
- Loomis, C.R.; Shipley, G.G.; Small, D.M. The phase behavior of hydrated cholesterol. J. Lipid Res. 1979, 20, 525–535. [Google Scholar] [CrossRef]
- Garti, N.; Karpuj, L.; Sarig, S. Correlation between crystal habit and the composition of solvated and nonsolvated cholesterol crystals. J. Lipid Res. 1981, 22, 785–791. [Google Scholar] [CrossRef]
- Kumbhar, D.D.; Pokharkar, V.B. Physicochemical investigations on an engineered lipid–polymer hybrid nanoparticle containing a model hydrophilic active, zidovudine. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 714–725. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. Z. Leb. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Prajakta, D.; Ratnesh, J.; Chandan, K.; Suresh, S.; Grace, S.; Meera, V.; Vandana, P. Curcumin Loaded pH-Sensitive Nanoparticles for the Treatment of Colon Cancer. J. Biomed. Nanotechnol. 2009, 5, 445–455. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A. Investigation by response surface methodology of the combined effect of pH and composition of water-methanol mixtures on the stability of curcuminoids. Food Chem. 2017, 219, 414–418. [Google Scholar] [CrossRef]
- Gao, J.; Ming, J.; He, B.; Fan, Y.; Gu, Z.; Zhang, X. Preparation and characterization of novel polymeric micelles for 9-nitro-20(S)-camptothecin delivery. Eur. J. Pharm. Sci. 2008, 34, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Lateh, L.; Kaewnopparat, N.; Yuenyongsawad, S.; Panichayupakaranant, P. Enhancing the water-solubility of curcuminoids-rich extract using a ternary inclusion complex system: Preparation, characterization, and anti-cancer activity. Food Chem. 2022, 368, 130827. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core-shell protein-polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, P.D.F.; Francisco, C.R.L.; Coqueiro, A.; Leimann, F.V.; Pinela, J.; Calhelha, R.C.; Porto Ineu, R.; Ferreira, I.C.F.R.; Bona, E.; Gonçalves, O.H. The nanoencapsulation of curcuminoids extracted from: Curcuma longa L. and an evaluation of their cytotoxic, enzymatic, antioxidant and anti-inflammatory activities. Food Funct. 2019, 10, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Vaz, V.M.; Jitta, S.R.; Verma, R.; Kumar, L. Hesperetin loaded proposomal gel for topical antioxidant activity. J. Drug Deliv. Sci. Technol. 2021, 66, 102873. [Google Scholar] [CrossRef]







| 1-Homogenization Technique 1 | N° Formulation | Emulsion Observed | EE (%) | ||
|---|---|---|---|---|---|
| Ultrasonic Processor One-time addition, 130 watts, 20 kHertz, 3 mm Ti probe | Sonication Time | 30 min | 1 | No | NA |
| 45 min | 2 | No | NA | ||
| High Speed Homogenizer 10,000 rpm, 10 min | Mixing phases speed | Slow | 3 | Yes | 46 |
| Medium | 4 | Yes | 98 | ||
| Fast | 5 | Yes | 30 | ||
| 2-Optimization of homogenization technique 1 | Emulsion observed | EE (%) | |||
| High speed homogenizer, mixing phases at medium speed, 10 min | Homogenization speed | 12,000 rpm | 6 | Yes | 92 |
| 16,000 rpm | 7 | Yes | 98 | ||
| 3-Lipid, drug loading, lipid-drug ratio, and organic solvent | Emulsion observed | EE (%) | |||
| Type of lipid | 24:1 lipid:drug ratio | Cocoa butter | 8 | Yes | 71 |
| Cetyl Palmitate | 9 | Yes | 54 | ||
| Stearic acid | 10 | Yes | 81 | ||
| Cholesterol: Cetyl Palmitate 1:1 | 11 | Yes | 47 | ||
| Drug loading | mg of CUR | 7.5 | 12 | Yes | 87 |
| 10 | 13 | Yes | 62 | ||
| 15 | 14 | No | NA | ||
| Lipid-drug ratio | Chol:CUR | 12:1 | 15 | Yes | 75 |
| 48:1 | 16 | Yes | 70 | ||
| Organic solvent | 1:1 solvent mixture | CH2Cl2:MeOH | 17 | Yes | 37 |
| CH3Cl3:EtOH | 18 | Yes | 8 | ||
| 4-Surfactant selection and polymer concentration | Emulsion observed | EE (%) | |||
| Surfactant selection | 1% surfactant concentration | Tween 80 | 19 | No | NA |
| Span 80 | 20 | No | NA | ||
| Polymer concentration | mg/mL of Pluronic F-127 | 2 | 21 | Yes | 5 |
| 7 | 22 | Yes | 16 | ||
| IC50 (µg/mL) 1,2,3 | |||
|---|---|---|---|
| EtOH | Water | PLHN | |
| DMC | 12.46 a,# ± 0.02 | 2143.07 a,& ± 0.61 | 12.74 a,# ± 0.09 |
| BDM | 17.94 b,^ ± 0.06 | 1398.68 b,* ± 5.07 | 16.03 b,^ ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelm Romero, K.; Quirós, M.I.; Vargas Huertas, F.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles. Polymers 2021, 13, 4207. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234207
Wilhelm Romero K, Quirós MI, Vargas Huertas F, Vega-Baudrit JR, Navarro-Hoyos M, Araya-Sibaja AM. Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles. Polymers. 2021; 13(23):4207. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234207
Chicago/Turabian StyleWilhelm Romero, Krissia, María Isabel Quirós, Felipe Vargas Huertas, José Roberto Vega-Baudrit, Mirtha Navarro-Hoyos, and Andrea Mariela Araya-Sibaja. 2021. "Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and In Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles" Polymers 13, no. 23: 4207. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234207