Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analysis
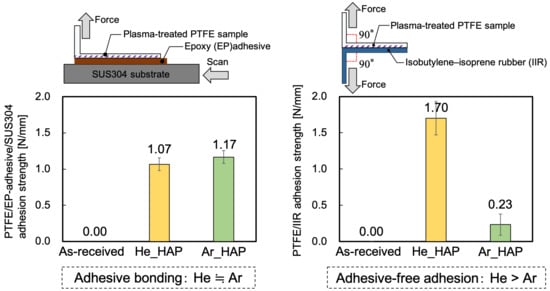
2.3.1. Evaluation of Adhesion Properties
2.3.2. Evaluation of Peroxy Radicals
2.3.3. Evaluation of Surface Chemical Composition
2.3.4. Measurement of Plasma Surface Modification Depth
2.3.5. Confirmation of Removal of Weak Boundary Layer (WBL)
2.3.6. Optical Emission Spectroscopy (OES) Measurements
3. Results and Discussion
3.1. Adhesion Properties
3.2. Peroxy Radical Density Ratio Calculated from ESR Spectra
3.3. Surface Chemical Composition Evaluated via XPS
3.4. Surface Modification Depth Evaluated via GCIB–XPS
3.5. Confirmation of WBL Removal by SEM Observation and Surface Hardness
3.6. Influence of VUV/UV Irradiation on the Generation of Peroxy Radicals and Surface Chemical Composition
3.7. Difference between He- and Ar-HAP Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murahara, M.; Toyoda, K. Excimer laser-induced photochemical modification and adhesion improvement of a fluororesin surface. J. Adhes. Sci. Technol. 1995, 9, 1601–1609. [Google Scholar] [CrossRef]
- Girardeaux, C.; Idrissi, Y.; Pireaux, J.J.; Caudano, R. Etching and functionalization of a fluorocarbon polymer by UV laser treatment. Appl. Surf. Sci. 1996, 96–98, 586–590. [Google Scholar] [CrossRef]
- Hopp, B.; Geretovszky, Z.; Bertóti, I.; Boyd, I.W. Comparative tensile strength study of the adhesion improvement of PTFE by UV photon assisted surface processing. Appl. Surf. Sci. 2002, 186, 80–84. [Google Scholar] [CrossRef]
- Nishi, Y.; Uyama, M.; Kawazu, H.; Takei, H.; Iwata, K.; Kudoh, H.; Mitsubayashi, K. Effects of electron beam irradiation on adhesive force of laminated sheet of high strength polytetrafluoroethylene (PTFE) and bio-adaptable polydimethylsiloxane (PDMS). Mater. Trans. 2012, 53, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Kubo, C.; Kanda, M.; Nishi, Y. Effects of homogeneous low energy electron beam irradiation (HLEBI) on adhesive force of peeling of carbonfiber reinforced epoxy polymer (CFRP) and polytetrafluoroethylene (PTFE). Mater. Trans. 2015, 56, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
- Utsumi, Y.; Yamamoto, S.; Kuroki, T.; Okubo, M. Direct bonding of PFTF sheets assisted by shynchrotron radiation induced surface modification. Microsyst. Technol. 2010, 16, 1495–1500. [Google Scholar] [CrossRef]
- Encinas, N.; Pantoja, M.; Torres-Remiro, M.; Martínez, M.A. Approaches to poly(tetrafluoroethylene) adhesive bonding. J. Adhes. 2011, 87, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, N.; Tasaka, S.; Umehara, T. Effects of surface modification by remote hydrogen plasma on adhesion in poly(tetrafluoroethylene)/copper composites. J. Appl. Polym. Sci. 1999, 71, 2191–2200. [Google Scholar] [CrossRef]
- Kim, S.R. Surface modification of poly(tetrafluoroethylene) film by chemical etching, plasma, and ion beam treatments. J. Appl. Polym. Sci. 2000, 77, 1913–1920. [Google Scholar] [CrossRef]
- Zou, X.P.; Kang, E.T.; Neoh, K.G.; Cui, C.Q.; Lim, T.B. Surface modification of poly(tetrafluoroethylene) films by plasma pre-activation and plasma polymerization of glycidyl methacrylate. Plasmas Polym. 2000, 5, 219–234. [Google Scholar] [CrossRef]
- Rodriguez-Santiago, V.; Bujanda, A.A.; Stein, B.E.; Pappas, D.D. Atmospheric plasma processing of polymers in helium-water vapor dielectric barrier discharges. Plasma Process. Polym. 2011, 8, 631–639. [Google Scholar] [CrossRef]
- Watari, K.; Iwao, T.; Yumoto, M. Structure change of PTFE by low-energy ion irradiation. Restraint of structure collapse by crosslinking structures. Electr. Eng. Jpn. 2012, 178, 1–7. [Google Scholar] [CrossRef]
- Nakayama, A.; Iwao, T.; Yumoto, M. Contribution of N2 ion on polar group introduction at PTFE surface by high E/n discharge. Electr. Eng. Jpn. 2012, 179, 1–7. [Google Scholar] [CrossRef]
- Takata, R.; Iwao, T.; Yumoto, M. Surface modification of PTFE using low-energy nitrogen ion irradiation: Improvement in adhesive strength on modification of deep modifying layer. Electron. Commun. Jpn. 2016, 99, 93–99. [Google Scholar] [CrossRef]
- Miller, M.L.; Postal, R.H.; Sawyer, P.N.; Martin, J.G.; Kaplit, M.J. Conditioning polytetrafluoroethylene surfaces for use in vascular prostheses. J. Appl. Polym. Sci. 1970, 14, 257–266. [Google Scholar] [CrossRef]
- Dwight, D.W.; Riggs, W.M. Fluoropolymer surface studies. J. Colloid Interface Sci. 1974, 47, 650–660. [Google Scholar] [CrossRef]
- Marchesi, J.T.; Keith, H.D.; Garton, A. Adhesion to sodium naphthalenide treated fluoropolymers. Part III. Mechanism of adhesion. J. Adhes. 1992, 39, 185–205. [Google Scholar] [CrossRef]
- Kang, E.T.; Zhang, Y. Surface modification of fluoropolymers via molecular design. Adv. Mater. 2000, 12, 1481–1494. [Google Scholar] [CrossRef]
- Zhang, M.C.; Kang, E.T.; Neoh, K.G.; Tan, K.L. Surface modification of aluminum foil and PTFE film by graft polymerization for adhesion enhancement. Colloids Surf. A Physicochem. Eng. Asp. 2001, 176, 139–150. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Dong, T. Studies on the adhesion between polytetrafluoroethylene film and silanized glass foil. Surf. Interface Anal. 2004, 36, 311–316. [Google Scholar] [CrossRef]
- Okubo, M.; Tahara, M.; Aburatani, Y.; Kuroki, T.; Hibino, T. Preparation of PTFE film with adhesive surface treated by atmospheric-pressure nonthermal plasma graft polymerization. IEEE Trans. Ind. Appl. 2010, 46, 1715–1721. [Google Scholar] [CrossRef]
- Kuroki, T.; Tahara, M.; Kuwahara, T.; Okubo, M. Microfabrication and metal plating technologies on polytetrafluoroethylene film surface treated by atmospheric-pressure nonthermal-plasma graft polymerization process. IEEE Trans. Ind. Appl. 2014, 50, 45–50. [Google Scholar] [CrossRef]
- Okubo, M.; Onji, T.; Kuroki, T.; Nakano, H.; Yao, E.; Tahara, M. Molecular-level reinforced adhesion between rubber and PTFE film treated by atmospheric plasma polymerization. Plasma Chem. Plasma Process. 2016, 36, 1431–1448. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ishihara, K.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Drastic improvement in adhesion property of polytetrafluoroethylene (PTFE) via heat-assisted plasma treatment using a heater. Sci. Rep. 2017, 7, 9476. [Google Scholar] [CrossRef]
- Primc, G. Recent advances in surface activation of polytetrafluoroethylene (PTFE) by gaseous plasma treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Comparison between adhesion properties of adhesive bonding and adhesive-free adhesion for heat-assisted plasma-treated polytetrafluoroethylene (PTFE). J. Adhes. 2020, 96, 776–796. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H. Japan Platform for Patent Information. Japan Patent 5,767,528, 26 June 2015. Available online: https://www.j-platpat.inpit.go.jp/web/PU/JPB_5767528/A0E7706779B69900134518459524470F (accessed on 28 September 2021).
- Zhang, Y.; Ishikawa, K.; Mozetič, M.; Tsutsumi, T.; Kondo, H.; Sekine, M.; Hori, M. Polyethylene terephthalate (PET) surface modification by VUV and neutral active species in remote oxygen or hydrogen plasmas. Plasma Process. Polym. 2019, 16, 1800175. [Google Scholar] [CrossRef]
- Wilson, D.J.; Williams, R.L.; Pond, R.C. Plasma modification of PTFE surfaces. Part I: Surfaces immediately following plasma treatment. Surf. Interface Anal. 2001, 31, 385–396. [Google Scholar] [CrossRef]
- Momose, Y.; Tamura, Y.; Ogino, M.; Okazaki, S.; Hirayama, M. Chemical reactivity between Teflon surfaces subjected to argon plasma treatment and atmospheric oxygen. J. Vac. Sci. Technol. A 1992, 10, 229–238. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectros. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Hubert, J.; Dufour, T.; Vandencasteele, N.; Desbief, S.; Lazzaroni, R.; Reniers, F. Etching processes of polytetrafluoroethylene surfaces exposed to he and He–O2 atmospheric post-discharges. Langmuir 2021, 28, 9466–9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkubo, Y.; Nakagawa, T.; Endo, K.; Yamamura, K. Influence of air contamination during heat-assisted plasma treatment on adhesion properties of polytetrafluoroethylene (PTFE). RSC Adv. 2019, 9, 22900–22906. [Google Scholar] [CrossRef] [Green Version]
- Massines, F.; Messaoudi, R.; Mayoux, C. Comparison between air filamentary and helium glow dielectric barrier discharges for the polypropylene surface treatment. Plasmas Polym. 1998, 3, 43–59. [Google Scholar] [CrossRef]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Tresp, H.; Hammer, M.U.; Weltmann, K.-D. Controlling the ambient air affected reactive species composition in the effluent of an argon plasma jet. IEEE Trans. Plasma Sci. 2012, 40, 2788–2794. [Google Scholar] [CrossRef]
- Jamil, M.T.; Ahmad, J.; Bukhari, S.H.; Mazhar, M.E.; Nissar, U.; Rao, A.J.; Ahmad, H.; Murtaza, G. Atmospheric pressure glow discharge (APGD) plasma generation and surface modification of aluminum and silicon Si (100). Dig. J. Nanomater. Biostruct. 2017, 12, 595–604. [Google Scholar]











| Sample | C [at.%] | O [at.%] | F [at.%] |
|---|---|---|---|
| As-received | 31.3 | 0.0 | 68.7 |
| He_HAP | 43.5 | 3.1 | 53.4 |
| Ar_HAP | 37.4 | 0.8 | 61.8 |
| Functional Group Type | CF2 | C–F | O–C=O | C=O | C–O | C–C |
|---|---|---|---|---|---|---|
| Binding energy [eV] | 291.8 | 289.8 | 289.2 | 288.0 | 286.5 | 285.3 |
| As-received [%] | 100 | 0 | 0 | 0 | 0 | 0 |
| He-HAP [%] | 55 | 4 | 6 | 5 | 17 | 12 |
| Ar-HAP [%] | 77 | 7 | 3 | 4 | 7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohkubo, Y.; Okazaki, Y.; Shibahara, M.; Nishino, M.; Seto, Y.; Endo, K.; Yamamura, K. Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE). Polymers 2021, 13, 4266. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234266
Ohkubo Y, Okazaki Y, Shibahara M, Nishino M, Seto Y, Endo K, Yamamura K. Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE). Polymers. 2021; 13(23):4266. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234266
Chicago/Turabian StyleOhkubo, Yuji, Yuki Okazaki, Masafumi Shibahara, Misa Nishino, Yosuke Seto, Katsuyoshi Endo, and Kazuya Yamamura. 2021. "Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE)" Polymers 13, no. 23: 4266. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234266