Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N,O-Carboxymethyl Chitosan (NOCC), Thiolated Hyaluronic Acid (HA-SH) and Dopa-Conjugated Thiolated Hyaluronic Acid (SH-HA-Dopa)
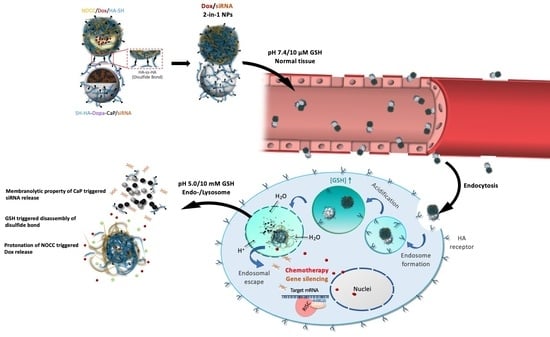
2.3. Preparation and Characterization of Dox/siRNA 2-in-1 Chimeric NPs
2.4. Drug Loading and Triggered Release
2.5. In Vitro Cytotoxicity
2.6. Cellular Uptake
2.7. In Vitro Therapeutic Activity
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of NOCC, HA-SH and SH-HA-Dopa
3.2. Formation of NOCC/Dox/HA-SH, SH-HA-Dopa-CaP/siRNA and Dox/siRNA 2-in-1 Chimeric NPs
3.3. In Vitro Cytotoxicity Studies
3.4. Release of Payloads in Response to pH and Redox Stimulation
3.5. Cellular Uptake, Internalization and Distribution
3.6. Antitumor Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gandhi, N.S.; Tekade, R.K. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: Current progress and advances. J. Control. Release 2014, 194, 238–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, T.; Wang, L. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef]
- Cheng, D.; Cao, N. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials 2012, 33, 1170–1179. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H. Bioreducible Cross-Linked Hyaluronic Acid/Calcium Phosphate Hybrid Nanoparticles for Specific Delivery of siRNA in Melanoma Tumor Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14576–14589. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Kudruk, S.; Chali, S.P. Biodegradable and Dual-Responsive Polypeptide-Shelled Cyclodextrin-Containers for Intracellular Delivery of Membrane-Impermeable Cargo. Adv. Sci. 2021, 8, 2100694–2100703. [Google Scholar] [CrossRef]
- Suo, A.; Qian, J. Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater. Sci. Eng. C 2017, 76, 659–672. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Lavasanifar, A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano 2011, 5, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, L. Chitosan-based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohyd. Polym. 2020, 238, 116126–116140. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120 Pt B, 1406–1419. [Google Scholar] [CrossRef]
- Jin, L.; Yoon, S.-J. Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration. Polymers 2021, 13, 3279. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bremner, D.H. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Vasantha, C.S. Layer-by-layer assembly of hyaluronic acid/carboxymethylchitosan polyelectrolytes on the surface of aminated mesoporous silica for the oral delivery of 5-fluorouracil. Eur. Polym. J. 2017, 93, 572–589. [Google Scholar] [CrossRef]
- Xiang, C.; Tenkumo, T. Gene transfection achieved by utilizing antibacterial calcium phosphate nanoparticles for enhanced regenerative therapy. Acta Biomater. 2021, 119, 375–389. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and Medical Applications of Calcium Phosphate Nanoparticles. Chemistry 2021, 27, 7471–7488. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Giger, E.V.; Castagner, B. siRNA transfection with calcium phosphate nanoparticles stabilized with PEGylated chelators. Adv. Healthc. Mater. 2013, 2, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Qiao, H. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials 2014, 35, 7978–7991. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.C. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sun, X. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.; Ku, S.H. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Lee, K.; Oh, M.H. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. Int. J. Pharm 2013, 445, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Yin, Y. Enhanced Transfection of Human Mesenchymal Stem Cells Using a Hyaluronic Acid/Calcium Phosphate Hybrid Gene Delivery System. Polymers 2019, 11, 798. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Wu, Y.C. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Sugimoto, M.; Morimotob, M. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohyd. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.E. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Control. Release 2014, 192, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-D.; Zhao, H.-Q. Novel hyaluronic acid–chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Mates, T.E. Metals and the integrity of a biological coating: The cuticle of mussel byssus. Langmuir 2009, 25, 3323–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, C. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Qiu, C.; Wei, W. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale 2016, 8, 13033–13044. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, X. Double-cross-linked hyaluronic acid nanoparticles with pH/reduction dual-responsive triggered release and pH-modulated fluorescence for folate-receptor-mediated targeting visualized chemotherapy. Biomacromolecules 2016, 17, 1496–1505. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Lai, C.-H. Targeting Tumor Cells with Nanoparticles for Enhanced Co-Drug Delivery in Cancer Treatment. Pharmaceutics 2021, 13, 1327. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H. Enzymatically Disulfide-Crosslinked Chitosan/Hyaluronic Acid Layer-by-Layer Self-Assembled Microcapsules for Redox-Responsive Controlled Release of Protein. ACS Appl. Mater. Interfaces 2018, 10, 33493–33506. [Google Scholar] [CrossRef]
- Li, J.; Huo, M. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, X. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-corona for dual suppression of drug resistance. Asian J. Pharm. Sci. 2020, 15, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Risnayanti, C.; Jang, Y.-S. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci. Rep. 2018, 8, 7498–7510. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Sample | Zeta Potential (mV) |
|---|---|
| NOCC/Dox/HA-SH | −30.8 ± 4.19 |
| SH-HA-Dopa-CaP/siRNA | −16.1 ± 1.71 |
| Dox/siRNA 2-in-1 NPs | −20.2 ± 6.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-C.; Kuo, W.-T. Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA. Polymers 2021, 13, 4362. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244362
Wu H-C, Kuo W-T. Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA. Polymers. 2021; 13(24):4362. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244362
Chicago/Turabian StyleWu, Hsi-Chin, and Wei-Ting Kuo. 2021. "Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA" Polymers 13, no. 24: 4362. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244362