Rice Hull-Derived Carbon for Supercapacitors: Towards Sustainable Silicon-Carbon Supercapacitors
Abstract
:1. Introduction
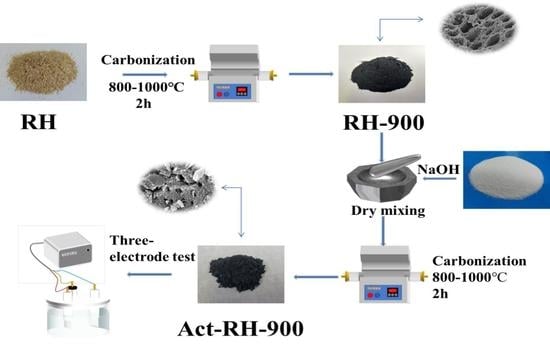
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Electrochemical Performance Tests
3. Results and Discussion
3.1. SEM
3.2. TEM
3.3. XRD and Raman
3.4. XPS
3.5. BET
3.6. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in Porous Organic Polymers: Syntheses, Structures, and Diverse Applications. Mater. Adv. 2022. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Atayde, E.C.; Matsagar, B.M.; Na, J.; Yamauchi, Y.; Wu, K.C.-W.; Kuo, S.-W. Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework. J. Taiwan Inst. Chem. Eng. 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Sun, G.; Qiu, L.; Zhu, M.; Kang, K.; Guo, X. Activated carbons prepared by hydrothermal pretreatment and chemical activation of Eucommia ulmoides wood for supercapacitors application. Ind. Crop. Prod. 2018, 125, 41–49. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Radhakrishnan, S.; Kim, S.-J. Supercapacitive properties of hydrothermally synthesized sphere like MoS2 nanostructures. Mater. Res. Bull. 2014, 50, 499–502. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef]
- Alaya, M.; Girgis, B.; Mourad, W. Activated Carbon from Some Agricultural Wastes Under Action of One-Step Steam Pyrolysis. J. Porous Mater. 2000, 7, 509–517. [Google Scholar] [CrossRef]
- Malik, P. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dyes Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Hsieh, Y.; Du, Y.; Jin, F.; Zhou, Z.; Enomoto, H. Alkaline pre-treatment of rice hulls for hydrothermal production of acetic acid. Chem. Eng. Res. Des. 2009, 87, 13–18. [Google Scholar] [CrossRef]
- Uzunova, S.A.; Uzunov, I.M.; Vassilev, S.V.; Alexandrova, A.K.; Staykov, S.G.; Angelova, D.B. Preparation of low-ash-content porous carbonaceous material from rice husks. Bulg. Chem. Commun. 2010, 42, 130–137. [Google Scholar]
- Novotvortsev, R.Y.; Suslova, E.V.; Chen, Q.; Akulich, A.N.; Lu, L.; Savilov, S.V. Supercapacitors Based on Activated Carbons, Products of Rice Hull Processing. Russ. J. Phys. Chem. A 2021, 95, 818–826. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2014, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, A.S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Peebles, L.H. Carbon Fibers: Formation, Structure, and Properties; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Sanji, T.; Fuchigami, A.; Tanaka, M. Synthesis and Photophysical Properties of Phenyleneethynylenes Containing a Combination of Two Main Group Element Moieties of B, Si, or P on the Side Chain. Organometallics 2018, 37, 350–358. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Xue, L.; Huang, T.; Yu, A. Carbon-coated SiO2 nanoparticles as anode material for lithium ion batteries. J. Power Sources 2011, 196, 10240–10243. [Google Scholar] [CrossRef]
- Demirbaş, A. Carbonization ranking of selected biomass for charcoal, liquid and gaseous products. Energy Convers. Manag. 2001, 42, 1229–1238. [Google Scholar] [CrossRef]
- Redondo, E.; Carretero-González, J.; Goikolea, E.; Ségalini, J.; Mysyk, R. Effect of pore texture on performance of activated carbon supercapacitor electrodes derived from olive pits. Electrochim. Acta 2015, 160, 178–184. [Google Scholar] [CrossRef]
- Bernatowicz, T.; Fraundorf, G.; Ming, T.; Anders, E.; Wopenka, B.; Zinner, E.; Fraundorf, P. Evidence for interstellar SiC in the Murray carbonaceous meteorite. Nature 1987, 330, 728–7300. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Kong, F.; Zhang, Y.; Wang, S.; Liu, S.; Lucia, L.; Fatehi, P.; Pang, H. Fabrication, characteristics and applications of carbon materials with different morphologies and porous structures produced from wood liquefaction: A review. Chem. Eng. J. 2019, 364, 226–243. [Google Scholar] [CrossRef]
- Guan, L.; Pan, L.; Peng, T.; Gao, C.; Zhao, W.; Yang, Z.; Hu, H.; Wu, M. Synthesis of Biomass-Derived Nitrogen-Doped Porous Carbon Nanosheests for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 8405–8412. [Google Scholar] [CrossRef]
- Ma, X.; Ding, C.; Li, D.; Wu, M.; Yu, Y. A facile approach to prepare biomass-derived activated carbon hollow fibers from wood waste as high-performance supercapacitor electrodes. Cellulose 2018, 25, 4743–4755. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Gai, S.; He, F.; Yang, P. Facile fabrication and electrochemical performance of flower-like Fe3O4@C@layered double hydroxide (LDH) composite. J. Mater. Chem. A 2014, 2, 8758–8765. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Shao, W.; Qin, W.; Zhao, X.; Kong, F. Facile fabrication and structure control of SiO2/carbon via in situ doping from liquefied bio-based sawdust for supercapacitor applications. Ind. Crop. Prod. 2020, 151, 112490. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.-W.; Lu, G.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Jianli, C.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A Fiber Supercapacitor with High Energy Density Based on Hollow Graphene/Conducting Polymer Fiber Electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Parikka, M. Global biomass fuel resources. Biomass Bioenergy 2004, 27, 613–620. [Google Scholar] [CrossRef]
- Selder, M.; Kadinski, L.; Makarov, Y.; Durst, F.; Wellmann, P.; Straubinger, T.; Hofmann, D.; Karpov, S.; Ramm, M. Global numerical simulation of heat and mass transfer for SiC bulk crystal growth by PVT. J. Cryst. Growth 2000, 211, 333–338. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, S.; Ding, Y.; Zhu, X.; Wang, Z.L.; Liu, M. Hierarchical Network Architectures of Carbon Fiber Paper Supported Cobalt Oxide Nanonet for High-Capacity Pseudocapacitors. Nano Lett. 2011, 12, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, H.; Yang, L.; Xiao, Y.; Zheng, M.; Liu, Y. High Specific Surface Area Rice Hull Based Porous Carbon Prepared for EDLCs. Int. J. Electrochem. Sci. 2012, 7, 4889–4897. [Google Scholar]
- Peng, H.; Zhou, J.; Sun, K.; Ma, G.; Zhang, Z.; Feng, E.; Lei, Z. High-Performance Asymmetric Supercapacitor Designed with a Novel NiSe@MoSe2 Nanosheet Array and Nitrogen-Doped Carbon Nanosheet. ACS Sustain. Chem. Eng. 2017, 5, 5951–5963. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; He, Y.-R.; Jiang, H.; Yu, H.-Q. High-Yield Harvest of Nanofibers/Mesoporous Carbon Composite by Pyrolysis of Waste Biomass and Its Application for High Durability Electrochemical Energy Storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Nam, K.S. High-resolution transmission electron microscopy study of solid phase crystallized silicon thin films on SiO2: Crystal growth and defects formation. J. Appl. Phys. 1995, 77, 95–102. [Google Scholar] [CrossRef]
- Mu, J.; Chen, B.; Guo, Z.; Zhang, M.; Zhang, Z.; Zhang, P.; Shao, C.; Liu, Y. Highly dispersed Fe3O4 nanosheets on one-dimensional carbon nanofibers: Synthesis, formation mechanism, and electrochemical performance as supercapacitor electrode materials. Nanoscale 2011, 3, 5034–5040. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Luo, J.; Chen, L. Highly nitrogen-doped graphitic carbon fibers from sustainable plant protein for supercapacitor. Ind. Crop. Prod. 2018, 121, 226–235. [Google Scholar] [CrossRef]
- Lee, J.-S.; Byeun, Y.-K.; Lee, S.-H.; Choi, S.-C. In situ growth of SiC nanowires by carbothermal reduction using a mixture of low-purity SiO2 and carbon. J. Alloys Compd. 2008, 456, 257–263. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Martins, V.L.; Smith, R.; Hall, P.J. Influence of Particle Size Distribution on the Performance of Ionic Liquid-based Electrochemical Double Layer Capacitors. Sci. Rep. 2016, 6, 22062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Luo, J.; Wang, X.; Sun, R. Lignin as a green reductant and morphology directing agent in the fabrication of 3D graphene-based composites for high-performance supercapacitors. Ind. Crop. Prod. 2017, 109, 410–419. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wei, T.-Y.; Chien, H.-C.; Lu, S.-Y. Manganese Oxide/Carbon Aerogel Composite: An Outstanding Supercapacitor Electrode Material. Adv. Energy Mater. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Characterization; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Lim, E.; Jo, C.; Lee, J. A mini review of designed mesoporous materials for energy-storage applications: From electric double-layer capacitors to hybrid supercapacitors. Nanoscale 2016, 8, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Kesavan, T.; Dash, S.S.; Sasidharan, M.; Sundramoorthy, A.K. MnO2 nanorods/SiO2 sphere coated on single-wall carbon nanotubes as supercapacitor electrode for high energy storage applications. Mater. Res. Express 2017, 4, 124004. [Google Scholar] [CrossRef]
- Ji, L.; Wang, B.; Yu, Y.; Wang, N.; Zhao, J. N, S co-doped biomass derived carbon with sheet-like microstructures for supercapacitors. Electrochim. Acta 2019, 331, 135348. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.-T.; Kuo, S.-W. Meso/Microporous Carbons from Conjugated Hyper-Crosslinked Polymers Based on Tetraphenylethene for High-Performance CO2 Capture and Supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Shao, W.; Wu, Q.; Zhao, X.; Kong, F. A new lamellar larch-based carbon material: Fabrication, electrochemical characterization and supercapacitor applications. Ind. Crop. Prod. 2020, 148, 112306. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Yang, H.; Li, Z.; Yu, C.; Tu, B.; Zhao, D. Ordered Mesoporous Polymers and Homologous Carbon Frameworks: Amphiphilic Surfactant Templating and Direct Transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef]
- Vilella, P.C.; Lira, J.A.; Azevedo, D.; Bastos-Neto, M.; Stefanutti, R. Preparation of biomass-based activated carbons and their evaluation for biogas upgrading purposes. Ind. Crop. Prod. 2017, 109, 134–140. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2@Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li-S Battery. Adv. Mater. 2016, 28, 3167–3172. [Google Scholar] [CrossRef]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Meng, T.-S.; Samy, M.M.; Kuo, S.-W. Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor. Polymers 2020, 12, 2426. [Google Scholar] [CrossRef] [PubMed]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Lu, Y.; Hua, X.; Liu, J.; Yang, Z.; Wei, H.; Mai, Y. All-organic covalent organic framework/polyaniline composites as stable electrode for high-performance supercapacitors. Mater. Lett. 2018, 236, 354–357. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Zhao, X.; Kong, F. Regulatory pore structure of biomass-based carbon for supercapacitor applications. Microporous Mesoporous Mater. 2020, 297, 110032. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; You, Y.; Xin, S.; Yin, Y.-X.; Zhang, J.; Wang, P.; Zheng, X.-S.; Cao, F.-F.; Guo, Y.-G. Rice husk-derived hierarchical silicon/nitrogen-doped carbon/carbon nanotube spheres as low-cost and high-capacity anodes for lithium-ion batteries. Nano Energy 2016, 25, 120–127. [Google Scholar] [CrossRef]
- Jiao, M.; Liu, K.; Shi, Z.; Wang, C. SiO2/Carbon Composite Microspheres with Hollow Core-Shell Structure as a High-Stability Electrode for Lithium-Ion Batteries. ChemElectroChem 2017, 4, 542–549. [Google Scholar] [CrossRef]









| Atom | C | O | Si | Others | |
|---|---|---|---|---|---|
| Sample | |||||
| RH-900 | 73.23 | 17.53 | 6.7 | 2.54 | |
| Act-RH-900 | 68.26 | 20.79 | 8.12 | 2.83 | |
| Sample | SBET (m² g−1) | Smicro/SBET (%) | Pore Volume (cm³ g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| RH-800 | 226 | 95 | 0.136 | 1.2 |
| RH-900 | 418 | 93 | 0.246 | 1.1 |
| RH-1000 | 286 | 77 | 0.224 | 1.7 |
| Act-RH-800 | 732 | 80 | 0.541 | 1.4 |
| Act-RH-900 | 768 | 83 | 0.563 | 1.4 |
| Act-RH-1000 | 229 | 65 | 0.511 | 4.4 |
| Materials | Electrolyte | Current Density (A g−1) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|
| Carbon fibers | Na2SO4 | 0.5 | 74 | [55] |
| M-NMCCs-1073 | KOH | 1 | 128 | [12] |
| Modified graphene | KOH | 0.5 | 135 | [23] |
| DPT-HPP | KOH | 0.5 | 110 | [30] |
| DAB-TFP COF | H2SO4 | 0.5 | 98 | [56] |
| TpPa-COF@PANI | H2SO4 | 0.2 | 95 | [57] |
| Act-RH-900 | KOH | 0.5 | 150.8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, H.; Zhang, L.; Jiao, S.; Zhang, H.; Zhang, J.; Li, P.; Tao, Y.; Zhao, X. Rice Hull-Derived Carbon for Supercapacitors: Towards Sustainable Silicon-Carbon Supercapacitors. Polymers 2021, 13, 4463. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244463
Li C, Chen H, Zhang L, Jiao S, Zhang H, Zhang J, Li P, Tao Y, Zhao X. Rice Hull-Derived Carbon for Supercapacitors: Towards Sustainable Silicon-Carbon Supercapacitors. Polymers. 2021; 13(24):4463. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244463
Chicago/Turabian StyleLi, Changwei, Honglei Chen, Liqiong Zhang, Shenghui Jiao, Huixin Zhang, Junliu Zhang, Peng Li, Yubo Tao, and Xin Zhao. 2021. "Rice Hull-Derived Carbon for Supercapacitors: Towards Sustainable Silicon-Carbon Supercapacitors" Polymers 13, no. 24: 4463. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244463