A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles
Abstract
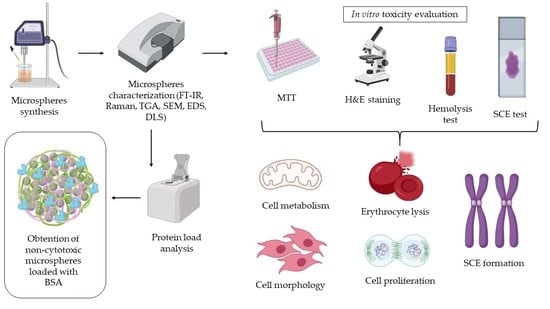
:1. Introduction
2. Materials and Methods
2.1. Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles Preparation
2.2. Nanoparticle Characterization
2.3. Cytotoxicity: MTT Assay and H&E Staining
2.4. Hemolysis Test
2.5. Sister Chromatid Exchange Assay
2.6. Surface Protein Load
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microsphere Characterization: FT-IR Spectroscopy
3.2. Microsphere Characterization: Raman Spectroscopy
3.3. Microsphere Characterization: TGA
3.4. Microsphere Characterization: SEM-EDS
3.5. Microspheres Characterization: DLS
3.6. Cytotoxicity of M-CS/Tween 80 Microspheres: MTT Assay
3.7. Cytotoxicity of M-CS Microspheres: H&E Staining
3.8. Hemolysis Assay
3.9. Sister Chromatid Exchange
3.10. Damage in The Proliferation Mechanism
3.11. Protein Load
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Calias, P.; Banks, W.A.; Begley, D.; Scarpa, M.; Dickson, P. Intrathecal delivery of pretein therapeutics to the brain: A critical reassessment. Pharmacol. Ther. 2014, 144, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Bi, H.; Wang, D.; Kang, M.; Tian, Z.; Zhang, Y.; Wang, H.; Zhu, T.; Ma, J. Preparation of Chitosan/Alginate-ellagic Acid Sustained-release Micro-spheres and their Inhibition of Preadipocyte Adipogenic Differentiation. Curr. Pharm. Biotechnol. 2019, 20, 1213–1222. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lv, Q.; Zhang, Q.; Zhu, H.; Liu, W.; Deng, G.; Wu, Y.; Shi, C.; Li, H.; Li, L. Preparation of carboxymethyl chitosan microspheres and their application in hemostasis. Disaster Med. Public Health Prep. 2017, 11, 660–667. [Google Scholar] [CrossRef]
- Shen, J.; Jin, B.; Qi, Y.C.; Jiang, Q.Y.; Gao, X.F. Carboxylated chitosan/silver-hydroxyapatite hybrid microspheres with improved antibacterial activity and cytocompatibility. Mater. Sci. Eng. C 2017, 78, 589–597. [Google Scholar] [CrossRef]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef]
- Yu, H.L.; Feng, Z.Q.; Zhang, J.J.; Wang, Y.H.; Ding, D.J.; Gao, Y.Y.; Zhang, W.F. The evaluation of proanthocyanidins/chitosan/lecithin microspheres as sustained drug delivery system. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Arthanari, S.; Mani, G.; Peng, M.M.; Jang, H.T. Chitosan-HPMC-blended microspheres as a vaccine carrier for the delivery of tetanus toxoid. Artif. Cells Nanomed. Biotechnol. 2016, 44, 517–523. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Berenjian, A.; Anarjan, N. Recent advances in application of chitosan in fuel cells. Sustain. Chem. Process. 2013, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mater. Sci. Eng. C 2017, 75, 947–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirala, N.R.; Harel, Y.; Lellouche, J.P.; Shtenberg, G. Ultrasensitive haptoglobin biomarker detection based on amplified chemiluminescence of magnetite nanoparticles. J. Nanobiotechnol. 2020, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghizadeh, S.; Ebrahiminezhad, A.; Ghoshoon, M.B. Magnetic immobilization of Pichia pastoris cells for the production of recombinant human serum albumin. Nanomaterials 2020, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frounchi, M.; Shamshiri, S. Magnetic nanoparticles-loaded PLA/PEG microspheres as drug carriers. J. Biomed. Mater. Res. Part A 2015, 103, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.J.; Jose, J.; Fratila, R.M.; Visscher, M.; Velders, A.H.; Ten, B. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media Mol. Imaging. 2013, 8, 83–91. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007, 52, 1653–1661. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jornsgard, H.; Tegnelius, D.; Erisson, S.; et al. The Nordic SentiMag trial: A comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, B.; Thiesen, B.; Feussener, A.; Von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neuroonco. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef]
- Yadav, M.; Parle, M.; Sharma, N.; Dhingra, S.; Raina, N. Brain targeted oral delivery of doxycycline hydrochloride encapsulated Tween 80 coated chitosan nanoparticles against ketamine induced psychosis: Behavioral, biochemical, neurochemical and histological alterations in mice. Drug Deliv. 2017, 24, 1429–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zheng, Y.; Zeng, X.; Jiang, L.; Chen, H.; Liu, R.; Huang, L.; Mei, L. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int. J. Nanomed. 2011, 6, 2679–2688. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaz, B.; Charron, G.; Pfeiffer, C.; Zhao, Y.; De la Fuente, J.M.; Liang, X.J.; Parak, W.J.; Del Pino, P. Interfacing engineered nanoparticles with biological systems: Anticipating adverse nano-bio interactions. Small 2013, 9, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Kang, S.G.; Xiu, P.; Zhao, Y.; Zhou, R. Interactions between proteins and carbon-based nanoparticles: Exploring the origin of nanotoxicity at the molecular level. Small 2013, 9, 1546–1556. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Wu, N.; Porter, D.; Buford, M.; Wolfarth, M.; Holian, A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol. 2009, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villareal, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite nanoparticles coated with PEG 3350-Tween 80: In vitro characterization using primary cell cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Sarkar, T.; Rawat, K.; Bohidar, H.B.; Solanki, P.R. Electrochemical immunosensor based on PEG capped iron oxide nanoparticles. J. Electroanal. Chem. 2016, 783, 208–216. [Google Scholar] [CrossRef]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.; Durrani, S.K.; Siddique, M.; Mehmood, M. Hydrothermal synthesis of alpha Fe2O3 nanoparticles capped by Tween-80. Mater. Lett. 2011, 65, 2224–2227. [Google Scholar] [CrossRef]
- Fu, X.; Kong, W.; Zhang, Y.; Jiang, L. Novel solid–solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage. RSC Adv. 2015, 5, 68881–68889. [Google Scholar] [CrossRef]
- Lotfi, S.; Ghaderi, F.; Bahari, A.; Mahjoub, S. Preparation and characterization of magnetite–chitosan nanoparticles and evaluation of their cytotoxicity effects on MCF7 and fibroblast cells. J. Supercond. Nov. Magn. 2017, 30, 3431–3438. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Jaramillo-Martínez, S.; Vargas-Requena, C.; Rodríguez-González, C.; Hernández-Santoyo, A.; Olivas-Armendáriz, I. Effect of extrapallial protein of Mytilus californianus on the process of in vitro biomineralization of chitosan scaffolds. Heliyon 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Maruthupandy, M.; Muneeswaran, T.; Anand, M.; Quero, F. Highly efficient multifunctional graphene/chitosan/magnetite nanocomposites for photocatalytic degradation of important dye molecules. Int. J. Biol. Macromol. 2020, 153, 736–746. [Google Scholar] [CrossRef]
- Zajac, A.; Hanuza, J.; Wandas, M.; Dyminska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim Acta A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Souza, N.L.G.D.; Salles, T.F.; Brandão, H.M.; Edwards, H.G.M.; Oliveira, L.F.C. Synthesis, vibrational spectroscopic and thermal properties of oxocarbon cross-linked chitosan. J. Braz. Chem. Soc. 2015, 26, 1247–1256. [Google Scholar] [CrossRef]
- Kishore, R.S.K.; Pappenberger, A.; Bauer-Dauphin, I.; Ross, A.; Buergi, B.; Staempfli, A.; Mahler, H.C. Degradation of polysorbates 20 and 80: Studies on thermal autoxidation and hydrolysis. J. Pharm. Sci. 2011, 100, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Fractionation of carboxylate anions from aqueous solution using chitosan cross-linked sorbent materials. J. Name 2013, 1–3. [Google Scholar] [CrossRef]
- Gomez-Perez, M.; Fourcade, L.; Mateescu, M.A.; Paquin, J. Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Anal. Biochem. 2017, 535, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Katrina, A.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Correia, S.I.; Pereira, H. Silva-Correia, J. VanDijk, C.N.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Current concepts: Tissue engineering and regenerative medicine applications in the ankle joint. J. R. Soc. Interface 2013, 11, 20130784. [Google Scholar] [CrossRef] [PubMed]
- Macías-Martínez, B.I.; Cortés-Hernández, D.A.; Zugasti-Cruz, A.; Cruz-Ortíz, B.R.; Múzquiz-Ramos, E.M. Heating ability and hemolysis test of magnetite nanoparticles obtained by a simple co-precipitation method. J. Appl. Res. Technol. 2016, 14, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Raga, O.; Rimantas, P. Genotoxic properties of Betonica officinalis, Gratiola officinalis, Vincetoxicum luteum and Vincetoxicum hirundinaria extracts. Food Chem. Toxicol. 2019, 134, 110815. [Google Scholar] [CrossRef]
- Turkez, H.; Yousef, M.I.; Sönmez, E.; Togar, B.; Bakan, F.; Soizo, P.; DiStefano, A. Evaluation of cytotoxic, oxidative stress and genotoxic responses of hydroxyapatite nanoparticles on human blood cells. J. Appl. Toxicol. 2014, 34, 373–379. [Google Scholar] [CrossRef]
- Buyukleyla, M.G.; Tuylu, B.A.; Sinan, H.; Sivas, H. The Genotoxic and Antigenotoxic Effects of Tannic Acid in Human Lymphocytes. Drug Chem. Toxicol. 2012, 35, 11–19. [Google Scholar] [CrossRef]
- Atlı-Şekeroğlu, Z.; Güneş, B.; Kontaş-Yedier, S.; Şekeroğlu, V.; Aydın, B. Effects of tartrazine on proliferation and genetic damage in human lymphocytes. Toxicol. Mech. Methods 2017, 27, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Chung-Lun, L.; Cheng-Huang, L.; Huan-Cheng, C.; Meng-Chih, S. Protein attachment on nanodiamonds. J. Phys. Chem. A 2015, 119, 7704–7711. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Berlina, A.N.; Ivanov, V.S.; Zherdev, A.V.; Dzantiev, B.B. Adsorption of proteins on gold nanoparticles: One or more layers? Colloids Surf. B Biointerfaces. 2019, 173, 557–563. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roacho-Pérez, J.A.; Rodríguez-Aguillón, K.O.; Gallardo-Blanco, H.L.; Velazco-Campos, M.R.; Sosa-Cruz, K.V.; García-Casillas, P.E.; Rojas-Patlán, L.; Sánchez-Domínguez, M.; Rivas-Estilla, A.M.; Gómez-Flores, V.; et al. A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles. Polymers 2021, 13, 400. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030400
Roacho-Pérez JA, Rodríguez-Aguillón KO, Gallardo-Blanco HL, Velazco-Campos MR, Sosa-Cruz KV, García-Casillas PE, Rojas-Patlán L, Sánchez-Domínguez M, Rivas-Estilla AM, Gómez-Flores V, et al. A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles. Polymers. 2021; 13(3):400. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030400
Chicago/Turabian StyleRoacho-Pérez, Jorge A, Kassandra O Rodríguez-Aguillón, Hugo L Gallardo-Blanco, María R Velazco-Campos, Karla V Sosa-Cruz, Perla E García-Casillas, Luz Rojas-Patlán, Margarita Sánchez-Domínguez, Ana M Rivas-Estilla, Víctor Gómez-Flores, and et al. 2021. "A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles" Polymers 13, no. 3: 400. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030400