Design of New Polyacrylate Microcapsules to Modify the Water-Soluble Active Substances Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Butyl Methacrylate–Methacrylic Acid (BUMA_MA), BUMA_MA_Methyl Methacrylate (MMA), BUMA_MA_MMA_Pentaerythritol Triacrylate (T3) and BUMA_Methacrylamide (MAC) Copolymers
2.3. Polymer Latex Characterization: Fourier Transform Infrared (FT-IR), Nuclear Magnetic Resonance (1H NMR), Dynamic Light Scattering (DLS) and Differential Scanning Calorimetry (DSC) Analyses
- heating from 0 °C to 100 °C at 20 °C min−1;
- 2 min isotherm at 100 °C;
- cooling from 100 °C to 0 °C at 20 °C min−1;
- 2 min isotherm at 0 °C;
- heating from 0 °C to 150 °C at 20 °C min−1.
2.4. Preparation Process for Methyl Orange (MO) Encapsulation within Polyacrylates Microcapsules and Scanning Electron Microscopy (SEM) Characterization
2.5. MO Kinetic Release
3. Results and Discussion
3.1. Synthesis and Characterization of BUMA-Based Lattices
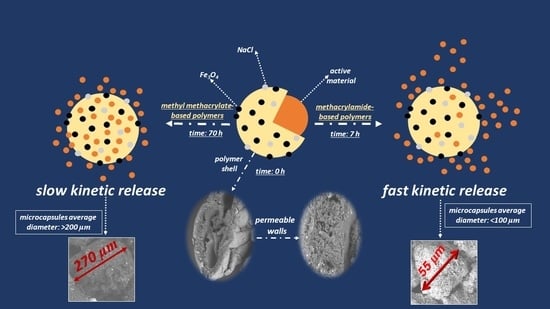
3.2. Pickering Emulsions and MO Kinetic Release of BUMA_MA_MMA_T3 and BUMA_MAC_75 Lattices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kissel, T.; Maretschek, S.; Packhauser, C.; Schnieders, J.; Seidel, N. Microencapsulation. Methods and Industrial Applications; CRC Press: Boca Raton, FL, USA; Taylor and Francis: New York, NY, USA, 2006. [Google Scholar]
- Siler-Marinkovic, S.; Bezbradica, D.; Skundric, P. Microencapsulation in the textile industry. Chem. Ind. Chem. Eng. Q. 2006, 12, 58–62. [Google Scholar] [CrossRef]
- Balassa, L.L.; Fanger, G.O.; Wurzburg, O.B. Microencapsulation in the food industry. CRC Crit. Rev. Food Technol. 1971, 2, 245–265. [Google Scholar] [CrossRef]
- Yeo, Y.; Baek, N.; Park, K. Microencapsulation methods for delivery of protein drugs. Biotechnol. Bioprocess Eng. 2001, 6, 213–230. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, Methods, Techniques, and Applications for Microencapsulated Phase Change Materials (MPCM): A Review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Scott, W.E.; Mcconnell, D.G. Microencapsulation Process. U.S. Patent 4956129A, 1981. [Google Scholar]
- Yow, H.N.; Routh, A.F. Formation of liquid core-polymer shell microcapsules. Soft Matter 2006, 2, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Rubner, M.F.; Cohen, R.E. Formation of nanoparticle-loaded microcapsules based on hydrogen-bonded multilayers. Chem. Mater. 2005, 17, 1099–1105. [Google Scholar] [CrossRef]
- Becker, A.L.; Zelikin, A.N.; Johnston, A.P.R.; Caruso, F. Tuning the formation and degradation of layer-by-layer assembled polymer hydrogel microcapsules. Langmuir 2009, 25, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Pan, T.; Li, H.; Huo, F.; Zheng, B.; Zhang, W. Encapsulation of Hydrophobic Guests within Metal–Organic Framework Capsules for Regulating Host–Guest Interaction. Chem. Mater. 2020, 32, 3553–3560. [Google Scholar] [CrossRef]
- Takahashi, R.; Miwa, S.; Rössel, C.; Fujii, S.; Lee, J.H.; Schacher, F.H.; Sakurai, K. Polymersome formation induced by encapsulation of water-insoluble molecules within ABC triblock terpolymers. Polym. Chem. 2020, 11, 3446–3452. [Google Scholar] [CrossRef]
- Brophy, M.R.; Deasy, P.B. Influence of coating and core modifications on the in vitro release of methylene blue from ethylcellulose microcapsules produced by pan coating procedure. J. Pharm. Pharmacol. 1981, 33, 495–499. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Microencapsulation by spray drying: Influence of emulsion size on the retention of volatile compounds. J. Food Sci. 2003, 68, 2256–2262. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Tsuda, N.; Ohtsubo, T.; Fuji, M. Preparation of self-bursting microcapsules by interfacial polymerization. Adv. Powder Technol. 2012, 23, 724–730. [Google Scholar] [CrossRef]
- Fan, C.; Zhou, X. Influence of operating conditions on the surface morphology of microcapsules prepared by in situ polymerization. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 363, 49–55. [Google Scholar] [CrossRef]
- Kobašlija, M.; McQuade, D.T. Polyurea microcapsules from oil-in-oil emulsions via interfacial polymerization. Macromolecules 2006, 39, 6371–6375. [Google Scholar] [CrossRef]
- Jinglei, Y.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of isocyanates for self-healing polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar]
- Brandau, T. Preparation of monodisperse controlled release microcapsules. Int. J. Pharm. 2002, 242, 179–184. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Tripathi, P.; Ubaidulla, U.; Anand, V. Design and development of gliclazide mucoadhesive microcapsules: In vitro and In vivo evaluation. AAPS PharmSciTech 2008, 9, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Ma, Y.; Hayat, K.; Jia, C.; Xia, S.; Zhang, X. Morphology and release profile of microcapsules encapsulating peppermint oil by complex coacervation. J. Food Eng. 2011, 104, 455–460. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, C.; Liu, H.; Wang, C.; Liu, X.; Tong, Z. Suspension polymerization based on inverse Pickering emulsion droplets for thermo-sensitive hybrid microcapsules with tunable supracolloidal structures. Polymer 2009, 50, 2587–2594. [Google Scholar] [CrossRef]
- Chatterjee, S.; Salaün, F.; Campagne, C.; Vaupre, S.; Beirão, A.; El-Achari, A. Synthesis and characterization of chitosan droplet particles by ionic gelation and phase coacervation. Polym. Bull. 2014, 71, 1001–1013. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Release of thyme oil from polylactide microcapsules. Ind. Eng. Chem. Res. 2011, 50, 13752–13761. [Google Scholar] [CrossRef]
- Rossier-Miranda, F.J.; Schroën, C.G.P.H.; Boom, R.M. Colloidosomes: Versatile microcapsules in perspective. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 343, 43–49. [Google Scholar] [CrossRef]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef]
- Li, J.; Stöver, H.D.H. Pickering emulsion templated layer-by-layer assembly for making microcapsules. Langmuir 2010, 26, 15554–15560. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Furusawa, K.; Nagayama, K. Assembly of latex particles by using emulsion droplets as templates. 2. Ball-like and composite aggregates. Langmuir 1996, 12, 2385–2391. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Xie, Y.; Zhang, H.; Yu, W.; Xiong, Y.; Xie, W.; Ma, X. Microencapsulation using natural polysaccharides for drug delivery and cell implantation. J. Mater. Chem. 2006, 16, 3252–3267. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, W.O.N.H.O.; Ha, W.A.N.S. Polyelectrolyte complexes of sodium alginate with chitosan or its derivatives for microcapsules. J. Appl. Polym. Sci. 1997, 425–432. [Google Scholar] [CrossRef]
- Czarnecki-Maulden, G.L. Effect of dietary modulation of intestinal microbiota on reproduction and early growth. Theriogenology 2008, 70, 286–290. [Google Scholar] [CrossRef]
- Lensen, D.; Vriezema, D.M.; van Hest, J.C.M. Polymeric microcapsules for synthetic applications. Macromol. Biosci. 2008, 8, 991–1005. [Google Scholar] [CrossRef]
- Arshady, R. Microspheres and microcapsules, a survey of manufacturing techniques Part II: Coacervation. Polym. Eng. Sci. 1990, 30, 905–914. [Google Scholar] [CrossRef]
- Sabatini, V.; Cattò, C.; Cappelletti, G.; Cappitelli, F.; Antenucci, S.; Farina, H.; Ortenzi, M.A.; Camazzola, S.; Di Silvestro, G. Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Prog. Org. Coat. 2018, 114, 47–57. [Google Scholar] [CrossRef]
- Sabatini, V.; Farina, H.; Montarsolo, A.; Pargoletti, E.; Ortenzi, M.A.; Cappelletti, G. Fluorinated Polyacrylic Resins for the Protection of Cultural Heritages: The Effect of Fluorine on Hydrophobic Properties and Photochemical Stability. Chem. Lett. 2018, 47, 280–283. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Comite, V.; Ortenzi, M.A.; Fermo, P.; Gulotta, D.; Cappelletti, G. Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study. Polymers 2019, 11, 1190. [Google Scholar] [CrossRef] [Green Version]
- Shchukin, D.G. Container-based multifunctional self-healing polymer coatings. Polym. Chem. 2013, 4, 4871–4877. [Google Scholar] [CrossRef] [Green Version]
- Gin, H.; Dupuy, B.; Caix, J.; Baquey, C.H.; Ducassou, D. In vitro diffusion in polyacrylamide embedded agarose microbeads. J. Microencapsul. 1990, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, B.; Cadic, C.; Gin, H.; Baquey, C.; Duty, B.; Ducassou, D. Microencapsulation of isolated pituitary cells by polyacrylamide microlatex coagulation on agarose beads. Biomaterials 1991, 12, 493–496. [Google Scholar] [CrossRef]
- Polk, A.; Amsden, B.; De Yao, K.; Peng, T.; Goosen, M.F.A. Controlled release of albumin from chitosan—Alginate microcapsules. J. Pharm. Sci. 1994, 83, 178–185. [Google Scholar] [CrossRef]
- Benita, S.; Hoffman, A.; Donbrow, M. Microencapsulation of paracetamol using polyacrylate resins (Eudragit Retard), kinetics of drug release and evaluation of kinetic model. J. Pharm. Pharmacol. 1985, 37, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Basuli, U.; Chattopadhyay, S.; Nah, C.; Chaki, T.K. Electrical Properties and Electromagnetic Interference Shielding Effectiveness of Multiwalled Carbon nanotubes—Reinforced EMA nanocomposites. Polym. Compos. 2012. [Google Scholar] [CrossRef]
- Santinho, A.J.P.; Ueta, J.M.; Freitas, O.; Pereira, N.L. Physicochemical characterization and enzymatic degradation of casein microcapsules prepared by aqueous coacervation. J. Microencapsul. 2002, 19, 549–558. [Google Scholar] [CrossRef] [PubMed]
- McMahon, W.A.; Lew, C.W.; Branly, K.L. Controlled Release Microcapsules. U.S. Patent 5466460A, 1995. [Google Scholar]
- Kydonieus, A.F. Controlled Release Technologies: Methods, Theory, and Applications; CRC Press: Boca Raton, FL, USA; Taylor and Francis: New York, NY, USA, 2019. [Google Scholar]
- Bashir, O.; Claverie, J.P.; Lemoyne, P.; Vincent, C. Controlled-release of Bacillus thurigiensis formulations encapsulated in lightresistant colloidosomal microcapsules for the management of lepidopteran pests of Brassica crops. PeerJ 2016, 2016, 1–9. [Google Scholar]
- Andersson Trojer, M.; Wendel, A.; Holmberg, K.; Nydén, M. The effect of pH on charge, swelling and desorption of the dispersant poly(methacrylic acid) from poly(methyl methacrylate) microcapsules. J. Colloid Interface Sci. 2012, 375, 213–215. [Google Scholar] [CrossRef]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J.; Ratoi, M.; Spikes, H.A. The effect of emulsifier concentration on the lubricating properties of oil-in-water emulsions. Tribol. Lett. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Olietti, A.; Pargoletti, E.; Diona, A.; Cappelletti, G. A novel optimized mold release oil-in-water emulsion for polyurethane foams production. J. Mol. Liq. 2018, 261, 199–207. [Google Scholar] [CrossRef]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef]
- Nonami, K. NII-Electronic Library Service. Chem. Pharm. Bull. 2002, 57, 364–370. [Google Scholar]
- Hayashi, M.; Furomoto, M. Microcapsules and Processes for Producing the Same. US20040195711A1, 2007. [Google Scholar]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer 2000, 41, 1657–1668. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y. FTIR and FT-raman studies of partially miscible poly(methyl methacrylate)/poly(4-vinylphenol) blends in solid states. Macromolecules 1997, 30, 286–292. [Google Scholar] [CrossRef]
- Braun, O.; Mallo, P. Novel Inverse Latex with a Low Content of Monomer Comprising a Strong Acid Functional Group and Use in the Manufacture of Topical Compositions. U.S. Patent No. 11/631,404, 15 November 2007. [Google Scholar]
- Huybrechts, J. Coatings Comprising Self-Stabilized Lattices Prepared in a Aqueous Carrier. U.S. Patent No. 5,936,026, 10 August 1999. [Google Scholar]
- Sathesh Prabu, C.; Thatheyus, A.J. Biodegradation of acrylamide employing free and immobilized cells of Pseudomonas aeruginosa. Int. Biodeterior. Biodegrad. 2007, 60, 69–73. [Google Scholar] [CrossRef]
- Yin, D.; Zhang, Q.; Yin, C.; Zhao, X.; Zhang, H. Hollow microspheres with covalent-bonded colloidal and polymeric shell by Pickering emulsion polymerization. Polym. Adv. Technol. 2012, 23, 273–277. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Wolfs, M.; Sellier, E.; Leal-Calderon, F.; Ravaine, V.; Schmitt, V. Soft microgels as Pickering emulsion stabilisers: Role of particle deformability. Soft Matter 2011, 7, 7689–7698. [Google Scholar] [CrossRef]
- Fox, T.G.; Loshaek, S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. J. Polym. Sci. 1955, 15, 371–390. [Google Scholar] [CrossRef]
- He, Y. Preparation and modification of ZnO microspheres using a Pickering emulsion as template. Mater. Lett. 2005, 59, 114–117. [Google Scholar] [CrossRef]
- Ekkehard, J.; Dieter, B.; Werner, B.; Peter, N. Microcapsule Preparations and Detergents and Cleaning Agents Containing Microcapsules. U.S. Patent No. 6,951,836, 4 October 2005. [Google Scholar]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Liao, L.P.; Wang, S.J.; Li, W.J. Self-healing coatings containing microcapsule. Appl. Surf. Sci. 2012, 258, 1915–1918. [Google Scholar] [CrossRef]





| Sample | MA/BUMA (mol mol−1) | MMA/BUMA (mol mol−1) | T3/BUMA (mol mol−1) | MAC/BUMA (mol mol−1) |
|---|---|---|---|---|
| BUMA_MA | 0.83 | - | - | - |
| BUMA_MA_MMA | 0.25 | 0.75 | - | - |
| BUMA_MA_MMA_T3 | 0.25 | 0.75 | 0.005 | - |
| BUMA_MAC_25 | - | - | - | 0.33 |
| BUMA_MAC_50 | - | - | - | 1.00 |
| BUMA_MAC_75 | - | - | - | 3.00 |
| Sample | <dDLS> (nm) | Fox Tg (°C) | Real Tg (°C) |
|---|---|---|---|
| BUMA_MA | 180 ± 70 | 67.1 | n.d. |
| BUMA_MA_MMA | (85 ± 15); (250 ± 60) | 55.5 | 78.2 |
| BUMA_MA_MMA_T3 | 80 ± 20 | 55.5 | 75.2 |
| BUMA_MAC_25 | (37 ± 7); (170 ± 30) | 40.8 | 46.1 |
| BUMA_MAC_50 | (25 ± 4); (80 ± 10) | 71.1 | 51.0 |
| BUMA_MAC_75 | 12 ± 3 | 120.3 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, V.; Pellicano, L.; Farina, H.; Pargoletti, E.; Annunziata, L.; Ortenzi, M.A.; Stori, A.; Cappelletti, G. Design of New Polyacrylate Microcapsules to Modify the Water-Soluble Active Substances Release. Polymers 2021, 13, 809. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050809
Sabatini V, Pellicano L, Farina H, Pargoletti E, Annunziata L, Ortenzi MA, Stori A, Cappelletti G. Design of New Polyacrylate Microcapsules to Modify the Water-Soluble Active Substances Release. Polymers. 2021; 13(5):809. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050809
Chicago/Turabian StyleSabatini, Valentina, Laura Pellicano, Hermes Farina, Eleonora Pargoletti, Luisa Annunziata, Marco A. Ortenzi, Alessandro Stori, and Giuseppe Cappelletti. 2021. "Design of New Polyacrylate Microcapsules to Modify the Water-Soluble Active Substances Release" Polymers 13, no. 5: 809. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050809