Importance of pH in Synthesis of pH-Responsive Cationic Nano- and Microgels
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Synthesis and Purification of Microgels
2.3. Photon Correlation Spectroscopy (PCS)
2.3.1. Angle-Dependent PCS
2.3.2. Temperature-Dependent PCS
2.4. Atomic Force Microscopy (AFM)
2.5. Nuclear Magnetic Resonace Spectroscopy
3. Results and Discussion
3.1. Variation of APMH-Feed
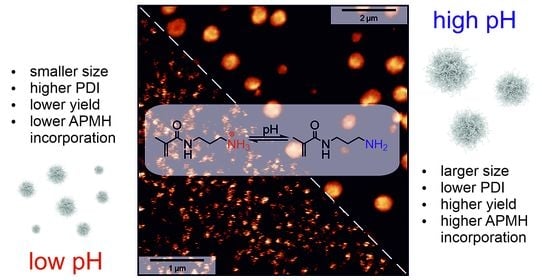
3.2. Variation of Reaction pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, W.O. Microgel, a New Macromolecule. Relation to Sol and Gel as Structural Elements of Synthetic Rubber. Rubber Chem. Technol. 1949, 22, 935–955. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Nayak, S.; Lyon, L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. 2005, 44, 7686–7708. [Google Scholar] [CrossRef]
- Döring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels–structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-iso. Colloids Surfaces 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Highly pH and Temperature Responsive Microgels Functionalized with Vinylacetic Acid. Macromolecules 2004, 37, 2544–2550. [Google Scholar] [CrossRef]
- Karg, M.; Pastoriza-Santos, I.; Rodriguez-González, B.; von Klitzing, R.; Wellert, S.; Hellweg, T. Temperature, pH, and ionic strength induced changes of the swelling behavior of PNIPAM-poly(allylacetic acid) copolymer microgels. Langmuir 2008, 24, 6300–6306. [Google Scholar] [CrossRef]
- Hu, X.; Tong, Z.; Lyon, L.A. Synthesis and Physicochemical Properties of Cationic Microgels Based on Poly(N-Isopropylmethacrylamide). Colloid Polym. Sci. 2010, 289, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedel, B.; Hertle, Y.; Wrede, O.; Bookhold, J.; Hellweg, T. Smart Homopolymer Microgels: Influence of the Monomer Structure on the Particle Properties. Polymers 2016, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Brändel, T.; Dirksen, M.; Hellweg, T. Tuning the Swelling Properties of Smart Multiresponsive Core-Shell Microgels by Copolymerization. Polymers 2019, 11, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Nessen, K.; Karg, M.; Hellweg, T. Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control. Polymer 2013, 54, 5499–5510. [Google Scholar] [CrossRef]
- Senff, H.; Richtering, W.; Norhausen, C.; Weiss, A.; Ballauff, M. Rheology of a Temperature Sensitive Core-Shell Latex. Langmuir 1999, 15, 102–106. [Google Scholar] [CrossRef]
- Hellweg, T. Responsive core-shell microgels: Synthesis, characterization, and possible applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1073–1083. [Google Scholar] [CrossRef]
- Watanabe, T.; Song, C.; Murata, K.; Kureha, T.; Suzuki, D. Seeded Emulsion Polymerization of Styrene in the Presence of Water-Swollen Hydrogel Microspheres. Langmuir 2018, 34, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, A.P.H.; Scotti, A.; Turnhoff, S.K.; Janssen, C.; Radulescu, A.; Pich, A.; Rudov, A.A.; Potemkin, I.I.; Richtering, W. An anionic shell shields a cationic core allowing for uptake and release of polyelectrolytes within core–shell responsive microgels. Soft Matter 2018, 14, 4287–4299. [Google Scholar] [CrossRef]
- Pich, A.; Karak, A.; Lu, Y.; Ghosh, A.K.; Adler, H.J.P. Tuneable Catalytic Properties of Hybrid Microgels Containing Gold Nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 3763–3769. [Google Scholar] [CrossRef]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147-148, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, E.D.; Faizuloev, E.B.; Izumrudov, V.A.; Litmanovich, E.A.; Melik-Nubarov, N.S. Synthesis of poly(N,N′-Dimethylaminoethyl Methacrylate) Nanogels Reverse Micelles Deliv. Plasmid DNA Small Interf. RNAs Living Cells. Polym. Sci. Ser. C 2012, 54, 69–79. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Hoare, T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3027–3043. [Google Scholar] [CrossRef]
- Islam, M.R.; Ahiabu, A.; Li, X.; Serpe, M.J. Poly (N-isopropylacrylamide) microgel-based optical devices for sensing and biosensing. Sensors 2014, 14, 8984–8995. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic polymer nanoparticles and nanogels: From synthesis to biotechnological applications. Chem. Rev. 2014, 114, 367–428. [Google Scholar] [CrossRef]
- Wellert, S.; Richter, M.; Hellweg, T.; von Klitzing, R.; Hertle, Y. Responsive Microgels at Surfaces and Interfaces. Z. Für Phys. Chem. 2015, 229. [Google Scholar] [CrossRef]
- Zhou, X.; Nie, J.; Du, B. Functionalized Ionic Microgel Sensor Array for Colorimetric Detection and Discrimination of Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 20913–20921. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, K.; Wegener, T.; Hertle, Y.; Bookhold, J.; Jaeger, M.; Hellweg, T.; Fery, A.; Duschl, C. Thermoresponsive Microgel Coatings as Versatile Functional Compounds for Novel Cell Manipulation Tools. Polymers 2018, 10, 656. [Google Scholar] [CrossRef] [Green Version]
- Höck, H.; Engel, S.; Weingarten, S.; Keul, H.; Schwaneberg, U.; Möller, M.; Bocola, M. Comparison of Candida antarctica Lipase B Variants for Conversion of -Caprolactone in Aqueous Medium—Part 2. Polymers 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Dirksen, M.; Dargel, C.; Meier, L.; Brändel, T.; Hellweg, T. Smart microgels as drug delivery vehicles for the natural drug aescin: Uptake, release and interactions. Colloid Polym. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, W.S.; Lee, C.; Zhang, Y.; Czarnecki, A.; Serpe, M.J. Probing the response of poly (N-isopropylacrylamide) microgels to solutions of various salts using etalons. J. Colloid Interface Sci. 2021, 585, 195–204. [Google Scholar] [CrossRef]
- Hellweg, T.; Henry-Toulmé, N.; Chambon, M.; Roux, D. Interaction of short DNA fragments with the cationic polyelectrolyte poly(ethylene imine): A dynamic light scattering study. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 163, 71–80. [Google Scholar] [CrossRef]
- Rossi, S.; Lorenzo-Ferreira, C.; Battistoni, J.; Elassari, A.; Pichot, C.; Delair, T. Polymer mediated peptide immobilization onto amino-containing N -isopropylacrylamide-styrene core-shell particles. Colloid Polym. Sci. 2004, 282, 215–222. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-functionalized nanogels for targeted siRNA delivery. Bioconjugate Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodlekere, P.; Cartelle, A.L.; Lyon, L.A. Design of functional cationic microgels as conjugation scaffolds. RSC Adv. 2016, 6, 31619–31631. [Google Scholar] [CrossRef]
- Nöth, M.; Hussmann, L.; Belthle, T.; El-Awaad, I.; Davari, M.D.; Jakob, F.; Pich, A.; Schwaneberg, U. MicroGelzymes: PH-Independent Immobilization of Cytochrome P450 BM3 in Microgels. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Eisold, S.; Alvarez, L.H.; Ran, K.; Hengsbach, R.; Fink, G.; Benigno, S.C.; Mayer, J.; Wöll, D.; Simon, U. DNA introduces an independent temperature responsiveness to thermosensitive microgels and enables switchable plasmon coupling as well as controlled uptake and release. Nanoscale 2021, 13, 2875–2882. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Reviews. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Reviews. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Maximova, E.D.; Zhiryakova, M.V.; Faizuloev, E.B.; Nikonova, A.A.; Ezhov, A.A.; Izumrudov, V.A.; Orlov, V.N.; Grozdova, I.D.; Melik-Nubarov, N.S. Cationic nanogels as Trojan carriers for disruption of endosomes. Colloids Surfaces. B Biointerfaces 2015, 136, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Mai-ngam, K.; Boonkitpattarakul, K.; Sakulsombat, M.; Chumningan, P.; Mai-ngam, B. Synthesis and phase separation of amine-functional temperature responsive copolymers based on poly(N-Isopropylacrylamide). Eur. Polym. J. 2009, 45, 1260–1269. [Google Scholar] [CrossRef]
- Xu, J.; Pelton, R. A new route to poly(N-isopropylacrylamide) microgels supporting a polyvinylamine corona. J. Colloid Interface Sci. 2004, 276, 113–117. [Google Scholar] [CrossRef]
- Farley, R.; Saunders, B.R. A general method for functionalisation of microgel particles with primary amines using click chemistry. Polymer 2014, 55, 471–480. [Google Scholar] [CrossRef]
- Thaiboonrod, S.; Berkland, C.; Milani, A.H.; Ulijn, R.; Saunders, B.R. Poly(vinylamine) microgels: PH-responsive particles with high primary amine contents. Soft Matter 2013, 9, 3920. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Pelton, R. Synthesis and Solution Properties of Poly(N-isopropylacrylamide-co-diallyldimethylammonium chloride). Macromolecules 1995, 28, 4617–4621. [Google Scholar] [CrossRef]
- Meunier, F.; Elaïssari, A.; Pichot, C. Preparation and characterization of cationic poly(N-Isopropylacrylamide) Copolymer Latexes. Polym. Adv. Technol. 1995, 6, 489–496. [Google Scholar] [CrossRef]
- Pinkrah, V.T.; Snowden, M.J.; Mitchell, J.C.; Seidel, J.; Chowdhry, B.Z.; Fern, G.R. Physicochemical Properties of Poly(N-Isopropylacrylamide Cationic Polyelectrolyte Colloid. Microgels. Langmuir 2003, 19, 585–590. [Google Scholar] [CrossRef]
- Gao, J.; Frisken, B.J. Influence of secondary components on the synthesis of self-cross-linked N-isopropylacrylamide microgels. Langmuir 2005, 21, 545–551. [Google Scholar] [CrossRef]
- Dubey, A.; Burke, N.A.D.; Stöver, H.D.H. Preparation and characterization of narrow compositional distribution polyampholytes as potential biomaterials: Copolymers of N -(3-aminopropyl)methacrylamide hydrochloride (APM) and methacrylic acid (MAA). J. Polym. Sci. Part A Polym. Chem. 2015, 53, 353–365. [Google Scholar] [CrossRef]
- Blauer, G. Polymerization of methacrylic acid at pH 4 to 11. Trans. Faraday Soc. 1960, 56, 606–612. [Google Scholar] [CrossRef]
- Riahinezhad, M.; McManus, N.; Penlidis, A. Effect of Monomer Concentration and pH on Reaction Kinetics and Copolymer Microstructure of Acrylamide/Acrylic Acid Copolymer. Macromol. React. Eng. 2015, 9, 100–113. [Google Scholar] [CrossRef]
- Zha, L.; Hu, J.; Wang, C.; Fu, S.; Elaissari, A.; Zhang, Y. Preparation and characterization of poly (N -isopropylacrylamide- co -dimethylaminoethyl methacrylate) microgel latexes. Colloid Polym. Sci. 2002, 280, 1–6. [Google Scholar] [CrossRef]
- Karanastasis, A.A.; Kenath, G.S.; Andersen, D.; Fokas, D.; Ryu, C.Y.; Ullal, C.K. One-pot surfactant-free modulation of size and functional group distribution in thermoresponsive microgels. J. Colloid Interface Sci. 2020, 568, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Frisken, B.J. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl. Opt. 2001, 40, 4087–4091. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.H.; Urquidi, J.; Singh, S.; Robinson, G.W. Thermal Offset Viscosities of Liquid H2O, D2O, and T2O. J. Phys. Chem. B 1999, 103, 1991–1994. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Ramos, J.; Forcada, J.; Martín-Molina, A. Computer simulations of thermo-sensitive microgels: Quantitative comparison with experimental swelling data. J. Chem. Phys. 2012, 136, 244903. [Google Scholar] [CrossRef]
- Truzzolillo, D.; Sennato, S.; Sarti, S.; Casciardi, S.; Bazzoni, C.; Bordi, F. Overcharging and reentrant condensation of thermoresponsive ionic microgels. Soft Matter 2018, 14, 4110–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, V. Encapsulation Nanotechnologies; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Gelissen, A.P.; Schmid, A.J.; Plamper, F.A.; Pergushov, D.V.; Richtering, W. Quaternized microgels as soft templates for polyelectrolyte layer-by-layer assemblies. Polymer 2014, 55, 1991–1999. [Google Scholar] [CrossRef]











| Microgel | APMH/mol% | pH | (10 °C)/nm | PDI/% | Yield/% | WSP/% |
|---|---|---|---|---|---|---|
| APMH-0 | 0.0 | 7.6 | 361 ± 18 | 4.6 ± 3.9 | 54 | 32 |
| APMH-2.5 | 2.5 | 3.3 | 110.7 ± 5.5 | 4.6 ± 4.1 | 41 | 37 |
| APMH-5 | 5.0 | 3.1 | 68.2 ± 3.4 | 9.1 ± 2.8 | 39 | 45 |
| APMH-10 | 10.0 | 2.6 | 37.7 ± 1.9 | 24.9 ± 3.5 | 24 | 73 |
| Microgel | APMH Content/% | Incorporation Success/% |
|---|---|---|
| APMH-0 | 0.0 | - |
| APMH-2.5 | 0.6 | 24 |
| APMH-5 | 1.2 | 24 |
| APMH-10 | 3.1 | 31 |
| Microgel | APMH/mol% | pH | (10 °C)/nm | PDI/% | yield/% | WSP/% |
|---|---|---|---|---|---|---|
| APMH-pH2.6 1 | 10.0 | 2.6 2 | 37.7 ± 1.9 | 24.9 ± 3.5 | 24 | 73 |
| APMH-pH7.0 | 10.0 | 7.0 | 42.8 ± 2.1 | 32.7 ± 3.1 | 28 | 63 |
| APMH-pH8.0 | 10.0 | 8.0 | 96.4 ± 4.8 | 7.0 ± 2.8 | 29 | 50 |
| APMH-pH8.5 | 10.0 | 8.5 | 215 ± 1.1 | 5.6 ± 3.3 | 28 | 47 |
| APMH-pH9.0 | 10.0 | 9.0 | 333 ± 17 | 4.4 ± 2.8 | 41 | 38 |
| APMH-pH9.5 | 10.0 | 9.5 | 493 ± 25 | 8.3 ± 4.5 | 47 | 35 |
| APMH-pH10.0 3 | 10.0 | 10.0 | - | - | 0 | - |
| APMH-pH10.5 3 | 10.0 | 10.5 | - | - | 0 | - |
| Microgel | APMH Content/% |
|---|---|
| APMH-pH2.6 | 3.1 |
| APMH-pH7.0 | 3.2 |
| APMH-pH8.0 | 6.1 |
| APMH-pH8.5 | 6.4 |
| APMH-pH9.0 | 7.5 |
| APMH-pH9.5 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annegarn, M.; Dirksen, M.; Hellweg, T. Importance of pH in Synthesis of pH-Responsive Cationic Nano- and Microgels. Polymers 2021, 13, 827. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050827
Annegarn M, Dirksen M, Hellweg T. Importance of pH in Synthesis of pH-Responsive Cationic Nano- and Microgels. Polymers. 2021; 13(5):827. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050827
Chicago/Turabian StyleAnnegarn, Marco, Maxim Dirksen, and Thomas Hellweg. 2021. "Importance of pH in Synthesis of pH-Responsive Cationic Nano- and Microgels" Polymers 13, no. 5: 827. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050827