Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating—Effect of the Base Paper and Nanoclay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
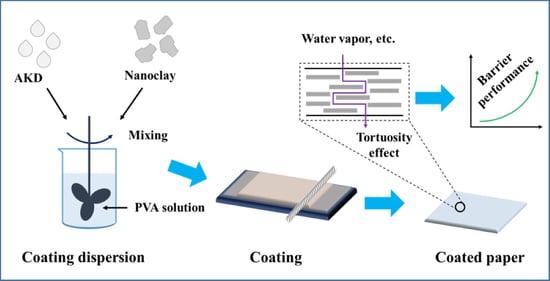
2.2.1. Preparation of Coating Dispersions
2.2.2. Coating Process
2.2.3. Confocal Laser Scanning Microscope (CLSM) Imaging
2.2.4. Surface Morphologies of Papers
2.2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.2.6. Measurements of the Water Contact Angle (WCA)
2.2.7. Measurements of the Cobb60 Value
2.2.8. Measurements of the WVTR and Grease Resistance
2.2.9. Mechanical Properties of Papers
3. Results and Discussion
3.1. Comparison of the Properties of the Two Base Papers
3.2. Effect of the Base Paper on the WVTR of Coated Papers
3.3. Analysis of the Surface Coverage and Chemical Interactions
3.4. Water Resistance and Barrier Performance of Coated Papers
3.5. Strength Properties of Coated Papers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domnica, D. Review concerning the functions of packaging. Land Forces Acad. Rev. 2010, 15, 44–48. [Google Scholar]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and mechanical properties of plasticized and cross-linked nanocellulose coatings for paper packaging applications. Cellulose 2017, 24, 3969–3980. [Google Scholar] [CrossRef]
- Tobjork, D.; Osterbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Alava, M.; Niskanen, K. The physics of paper. Rep. Prog. Phys. 2006, 69, 669. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Khelifi, B.; Bras, J. Impact of different coating processes of microfibrillated cellulose on the mechanical and barrier properties of paper. J. Mater. Sci. 2014, 49, 2879–2893. [Google Scholar] [CrossRef]
- Mazhari Mousavi, S.M.; Afra, E.; Tajvidi, M.; Bousfield, D.W.; Dehghani-Firouzabadi, M. Cellulose nanofiber/carboxymethyl cellulose blends as an efficient coating to improve the structure and barrier properties of paperboard. Cellulose 2017, 24, 3001–3014. [Google Scholar] [CrossRef]
- Hult, E.-L.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crops Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- Shen, Z.; Kwon, S.; Oh, K.; Abhari, A.R.; Lee, H.L. Facile fabrication of hydrophobic cellulosic paper with good barrier properties via PVA/AKD dispersion coating. Nord. Pulp Pap. Res. J. 2019, 34, 516–524. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. Tortuosity model to predict the combined effects of crystallinity and nano-sized clay mineral on the water vapour barrier properties of polylactic acid. Appl. Clay Sci. 2017, 141, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Mirmehdi, S.; Hein, P.R.G.; de Luca Sarantópoulos, C.I.G.; Dias, M.V.; Tonoli, G.H.D. Cellulose nanofibrils/nanoclay hybrid composite as a paper coating: Effects of spray time, nanoclay content and corona discharge on barrier and mechanical properties of the coated papers. Food Packag. Shelf Life 2018, 15, 87–94. [Google Scholar] [CrossRef]
- Chang, J.-H. Comparative analysis of properties of PVA composites with various nanofillers: Pristine clay, organoclay, and functionalized graphene. Nanomaterials 2019, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Mokhothu, T.H.; John, M.J. Review on hygroscopic aging of cellulose fibres and their biocomposites. Carbohydr. Polym. 2015, 131, 337–354. [Google Scholar] [CrossRef]
- Ozaki, Y. Application of confocal laser scanning microscopy (CLSM) for observing adhesives in paper. J. Adhes. Sci. Technol. 2011, 25, 723–741. [Google Scholar] [CrossRef]
- Park, H.; Park, S.Y.; Yook, S.; Kim, T.-Y.; Youn, H.J. Impregnation of paper with cellulose nanofibrils and polyvinyl alcohol to enhance durability. Nord. Pulp Pap. Res. J. 2020, 35, 106–114. [Google Scholar] [CrossRef]
- Seo, M.S.; Youn, H.J.; Lee, H.L. Penetration control of surface sizing starch using cationic PAM and its effect on the bending stiffness of paper. BioResources 2020, 15, 5489–5502. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. Polyvinyl alcohol as the barrier layer in thin film composite nanofiltration membranes: Preparation, characterization, and performance evaluation. J. Colloid Interface Sci. 2009, 338, 121–127. [Google Scholar] [CrossRef]
- Song, X.; Chen, F.; Liu, F. Preparation and characterization of alkyl ketene dimer (AKD) modified cellulose composite membrane. Carbohydr. Polym. 2012, 88, 417–421. [Google Scholar] [CrossRef]
- Song, X.; Chen, F.; Liu, F. Study on the reaction of alkyl ketene dimer (AKD) and cellulose fiber. BioResources 2012, 7, 652–662. [Google Scholar]
- Mallakpour, S.; Dinari, M. Biomodification of cloisite Na+ with L-methionine amino acid and preparation of poly(vinyl alcohol)/organoclay nanocomposite films. J. Appl. Polym. Sci. 2012, 124, 4322–4330. [Google Scholar] [CrossRef]
- Lee, K.H.; Youn, H.J.; Lee, H.L. UV/Vis spectrometry-based analysis of alkyl ketene dimer (AKD) retention to solve the waxy spot problem in the papermaking process. ACS Omega 2020, 5, 11227–11234. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Huh, C.; Mason, S. An experimental study of some effects of solid surface roughness on wetting. Colloids Surf. 1980, 1, 79–104. [Google Scholar] [CrossRef]
- Hu, Z.; Zen, X.; Gong, J.; Deng, Y. Water resistance improvement of paper by superhydrophobic modification with microsized CaCO3 and fatty acid coating. Colloids Surf. A Physicochem. Eng. Asp. 2009, 351, 65–70. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Ismail, A.; Rahim, N.; Mustafa, A.; Matsuura, T.; Ng, B.; Abdullah, S.; Hashemifard, S. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Baird, D.G. Preparation of polymer–clay nanocomposites and their properties. Adv. Polym. Technol. 2006, 25, 270–285. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Witt, M.A.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Mechanically improved polyvinyl alcohol-composite films using modified cellulose nanowhiskers as nano-reinforcement. Carbohydr. Polym. 2018, 191, 25–34. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Pratima, B. Paper and Its Properties. In Biermann’s Handbook of Pulp and Paper, 3rd ed.; Pratima, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–63. [Google Scholar]
- Awada, H.; Bouatmane, M.; Daneault, C. High strength paper production based on esterification of thermomechanical pulp fibers in the presence of poly (vinyl alcohol). Heliyon 2015, 1, e00038. [Google Scholar] [CrossRef] [Green Version]
- Hamzeh, Y.; Sabbaghi, S.; Ashori, A.; Abdulkhani, A.; Soltani, F. Improving wet and dry strength properties of recycled old corrugated carton (OCC) pulp using various polymers. Carbohydr. Polym. 2013, 94, 577–583. [Google Scholar] [CrossRef]










| Base Paper | Surface-Unsized | Surface-Sized |
|---|---|---|
| Basis weight (g/m2) Thickness (μm) | 84 | 81 |
| 99.25 | 97.12 | |
| Bulk (cm3/g) | 1.18 | 1.20 |
| Density (g/cm3) | 0.85 | 0.83 |
| PPS 1 roughness (μm) | 4.53 | 5.46 |
| Brightness (%) | 92.28 | 92.81 |
| WVTR 2 (g/m2∙day) | 543.11 | 533.01 |
| Coating | #1 | #2 | #3 | #4 | |
|---|---|---|---|---|---|
| Component | |||||
| PVA (pph 1) | 100 | 100 | 100 | 100 | |
| AKD (pph) | 15 | 15 | 15 | 15 | |
| Nanoclay (pph) | 0 | 3 | 5 | 10 | |
| Coated Paper | Single Coating | Double Coating | |
|---|---|---|---|
| Formulation | |||
| PVA/AKD | 6.51 ± 0.17 1 | 11.79 ± 0.16 | |
| PVA/AKD/3% nanoclay | 6.55 ± 0.08 | 12.28 ± 0.17 | |
| PVA/AKD/5% nanoclay | 6.52 ± 0.16 | 12.08 ± 0.16 | |
| PVA/AKD/10% nanoclay | 6.62 ± 0.16 | 12.46 ± 0.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Rajabi-Abhari, A.; Oh, K.; Yang, G.; Youn, H.J.; Lee, H.L. Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating—Effect of the Base Paper and Nanoclay. Polymers 2021, 13, 1334. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081334
Shen Z, Rajabi-Abhari A, Oh K, Yang G, Youn HJ, Lee HL. Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating—Effect of the Base Paper and Nanoclay. Polymers. 2021; 13(8):1334. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081334
Chicago/Turabian StyleShen, Zhenghui, Araz Rajabi-Abhari, Kyudeok Oh, Guihua Yang, Hye Jung Youn, and Hak Lae Lee. 2021. "Improving the Barrier Properties of Packaging Paper by Polyvinyl Alcohol Based Polymer Coating—Effect of the Base Paper and Nanoclay" Polymers 13, no. 8: 1334. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081334