Synthesis and Characterization of Biocompatible Methacrylated Kefiran Hydrogels: Towards Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Kefiran Polysaccharides
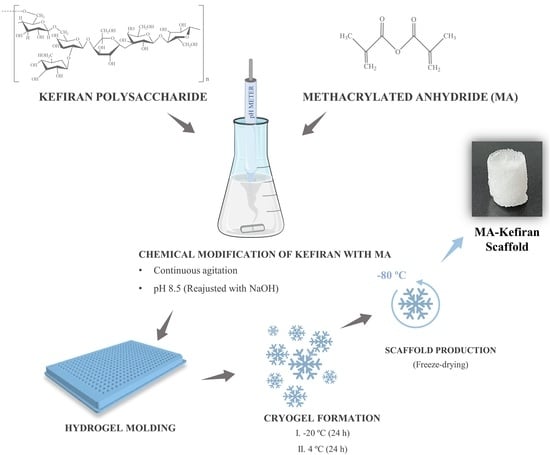
2.2. Synthesis of Methacrylated Kefiran
2.3. Preparation of MA-Kefiran Scaffold
2.4. Physicochemical Characterization of MA-Kefiran
2.4.1. H nuclear Magnetic Resonance Spectroscopy (NMR)
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.3. Gel Permeation Chromatography—Size Exclusion Chromatography (GPC-SEC)
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. Rheology
2.4.6. Injectability Assay
2.4.7. Micro-Computed Tomography (Micro-CT) Analysis
2.4.8. Scanning Electron Microscopy (SEM)
2.5. Biological Characterization of MA-Kefiran
2.5.1. Hemolytic Assay
2.5.2. Cytotoxicity Assessment
2.6. Statistical Analysis
3. Results
3.1. H-NMR
3.2. FTIR
3.3. DSC
3.4. GPC-SEC
3.5. Rheology of MA-Kefiran Extract
3.6. Injectability Assay
3.7. Production of MA-Kefiran Scaffolds
3.8. Assessment of the MA-Kefiran Scaffolds’ Morphological Properties
3.8.1. Micro-CT
3.8.2. SEM
3.9. Rheology of MA-Kefiran Cryogel
3.10. Biocompatibility of MA-Kefiran Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dlaska, C.E.; Andersson, G.; Brittberg, M.; Suedkamp, N.P.; Raschke, M.J.; Schuetz, M.A. Clinical Translation in Tissue Engineering—The Surgeon’s View. Curr. Mol. Biol. Rep. 2015, 1, 61–70. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum Hydrogels with Tunable Physical and Mechanical Properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials (Basel) 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisano, R.; Barresi, A.A.; Fissore, D. Innovation in Monitoring Food Freeze Drying. Dry. Technol. 2011, 29, 1920–1931. [Google Scholar] [CrossRef] [Green Version]
- Selhuber-Unkel, C.; Erdmann, T.; López-García, M.; Kessler, H.; Schwarz, U.S.; Spatz, J.P. Cell Adhesion Strength Is Controlled by Intermolecular Spacing of Adhesion Receptors. Biophys. J. 2010, 98, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, K.; Chaturvedi, K.; More, U.A.; Nadagouda, M.N.; Aminabhavi, T.M. Polysaccharide-Based Micro/Nanohydrogels for Delivering Macromolecular Therapeutics. J. Control. Release 2014, 193, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the Design of Macroporous Polymer Scaffolds for Potential Applications in Dentistry. J. Periodontal Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Porous Polymeric Structures for Tissue Engineering Prepared by a Coagulation, Compression Moulding and Salt Leaching Technique. Biomaterials 2003, 24, 1937–1947. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A Novel Fabrication Method of Macroporous Biodegradable Polymer Scaffolds Using Gas Foaming Salt as a Porogen Additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.A.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; Amjid Afridi, M.; Soo Han, S.; et al. Advances in the Scaffolds Fabrication Techniques Using Biocompatible Polymers and Their Biomedical Application: A Technical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A New Tool to Produce Alginate-Based Aerogels for Medical Applications, by Supercritical Gel Drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, N.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Synthesis and Properties of Hydrogels Prepared by Various Polymerization Reaction Systems. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. ISBN 978-3-319-76573-0. [Google Scholar]
- Radhouani, H.; Bicho, D.; Gonçalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran Cryogels as Potential Scaffolds for Drug Delivery and Tissue Engineering Applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Biological Performance of a Promising Kefiran-Biopolymer with Potential in Regenerative Medicine Applications: A Comparative Study with Hyaluronic Acid. J. Mater. Sci. Mater. Med. 2018, 29, 124. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Ahmad, A.; Khan, S.T.; Nisa, M.; Ahmad, H.; Afreen, A. Critical Reviews in Food Science and Nutrition Kefir and Health: A Contemporary Perspective Kefir and Health: A Contemporary Perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 422–434. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Kefiran Biopolymer: Evaluation of Its Physicochemical and Biological Properties. J. Bioact. Compat. Polym. 2018, 33, 461–478. [Google Scholar] [CrossRef]
- Merkx, D.W.H.; Westphal, Y.; van Velzen, E.J.J.; Thakoer, K.V.; de Roo, N.; van Duynhoven, J.P.M. Quantification of Food Polysaccharide Mixtures by 1H NMR. Carbohydr. Polym. 2018, 179, 379–385. [Google Scholar] [CrossRef]
- Dutta, S.; Burkhardt, K.; Bluhm, W.F.; Berman, H.M. Using the Tools and Resources of the RCSB Protein Data Bank. Curr. Protoc. Bioinformatics 2007, 20, 1.9.1–1.9.24. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Deshmukh, A.S. Polymeric Hydrogels as Smart Biomaterials; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-25320-6. [Google Scholar]
- Saba, N.; Jawaid, M.; Sultan, M.T.H. Thermal properties of oil palm biomass based composites. In Lignocellulosic Fibre and Biomass-Based Composite Materials: Processing, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2017; ISBN 9780081009666. [Google Scholar]
- Sabatino, M.A.; Carfì Pavia, F.; Rigogliuso, S.; Giacomazza, D.; Ghersi, G.; La Carrubba, V.; Dispenza, C. Development of Injectable and Durable Kefiran Hydro-Alcoholic Gels. Int. J. Biol. Macromol. 2020, 149, 309–319. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of Molecular Weight and Molecular Weight Distribution on Mechanical Properties of Polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Dragostin, O.; Profire, L. Molecular weight of polymers used in biomedical applications. In Characterization of Polymeric Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; ISBN 9780081007372. [Google Scholar]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Bhadu, G.R.; Kumar, S.; Choudhary, V. Rheological, Melting and Crystallization Behaviour of an Open Cage POSS/PTT Nanocomposite Prepared by Melt Blending. Int. J. Plast. Technol. 2010, 14, 7–23. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Della Sala, F.; Ambrosio, L.A. Rheometry of polymeric biomaterials. In Characterization of Polymeric Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; ISBN 9780081007372. [Google Scholar]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability Evaluation: An Open Issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent Advances in Hyaluronic Acid Based Therapy for Osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapasvi, S.; Mohanty, S.S.; Vedavyasa Acharya, K.K.; Bhattacharya, K.; Easwaran, R.; Charugulla, S.N. Viscosupplementation for Management of Knee Osteoarthritis from an Indian Perspective: An Expert Consensus Report. Pain Ther. 2019, 8, 217–231. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Bertoldi, S.; Farè, S.; Tanzi, M.C. Assessment of Scaffold Porosity: The New Route of Micro-CT. J. Appl. Biomater. Biomech. 2011, 9, 165–175. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT—A Digital 3D Microstructural Voyage into Scaffolds: A Systematic Review of the Reported Methods and Results. Biomater. Res. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng.-Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan Scaffolds: Interconnective Pore Size and Cartilage Engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnes, M.; Ratner, B.D.; Giachelli, C.M. A Fibrinogen Based Precision Microporous Scaffold For Tissue Engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of Synthetic Membranes Directed by Membrane Microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517–1524. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Xu, H.; Hu, K.; Sun, J.; Liu, M.; Zhang, F.; Gu, N. Pre-Vascularization in Fibrin Gel/PLGA Microsphere Scaffolds Designed for Bone Regeneration. NPG Asia Mater. 2018, 10, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and Characterization of a Model Extracellular Matrix That Induces Partial Regeneration of Adult Mammalian Skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Gorth, D.; Webster, T.J. Matrices for tissue engineering and regenerative medicine. In Biomaterials for Artificial Organs; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 270–286. ISBN 9781845696535. [Google Scholar]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen-Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Bružauskait, I.; Bironait, D.; Bagdonas Eiva Bernotien, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes-Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Gashti, M.P.; Alimohammadi, F.; Hulliger, J.; Burgener, M.; Oulevey-Aboulfad, H.; Bowlin, G.L. Microscopic Methods to Study the Structure of Scaffolds in Bone Tissue Engineering: A Brief Review. Curr. Microsc. Contrib. Adv. Sci. Technol. 2012, 1, 625–638. [Google Scholar]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of Freezing Rate on Pore Structure in Freeze-Dried Collagen-GAG Scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous Nanocellulose Gels and Foams: Breakthrough Status in the Development of Scaffolds for Tissue Engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials (Basel) 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; Ronca, A.; D’Amora, U.; Negri, G.; Ronca, D.; Ambrosio, L. Viscoelastic Properties of Rapid Prototyped Magnetic Nanocomposite Scaffolds for Osteochondral Tissue Regeneration. Procedia CIRP 2016, 49, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Schettini, N.; Jaroszeski, M.J.; West, L.; Saddow, S.E. Hemocompatibility Assessment of 3C-SiC for Cardiovascular Applications. In Silicon Carbide Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780123859068. [Google Scholar]
- Costa-Pinto, A.; Santos, T.C.; Neves, N.M.; Reis, R.L. Testing Natural Biomaterials in Animal Models. In Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119126218. [Google Scholar]
- Kobayashi, K.; Amiel, M.; Harwood, F.L.; Healey, R.M.; Sonoda, M.; Moriya, H.; Amiel, D. The Long-Term Effects of Hyaluronan during Development of Osteoarthritis Following Partial Meniscectomy in a Rabbit Model. Osteoarthr. Cartil. 2000, 8, 359–365. [Google Scholar] [CrossRef] [Green Version]







| Sample | ASTM Classification (%) |
|---|---|
| MA-Kefiran | 1.33 ± 0.21 |
| Kefiran | 2.00 ± 0.09 |
| PBS | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhouani, H.; Correia, S.; Gonçalves, C.; Reis, R.L.; Oliveira, J.M. Synthesis and Characterization of Biocompatible Methacrylated Kefiran Hydrogels: Towards Tissue Engineering Applications. Polymers 2021, 13, 1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081342
Radhouani H, Correia S, Gonçalves C, Reis RL, Oliveira JM. Synthesis and Characterization of Biocompatible Methacrylated Kefiran Hydrogels: Towards Tissue Engineering Applications. Polymers. 2021; 13(8):1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081342
Chicago/Turabian StyleRadhouani, Hajer, Susana Correia, Cristiana Gonçalves, Rui L. Reis, and Joaquim M. Oliveira. 2021. "Synthesis and Characterization of Biocompatible Methacrylated Kefiran Hydrogels: Towards Tissue Engineering Applications" Polymers 13, no. 8: 1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081342