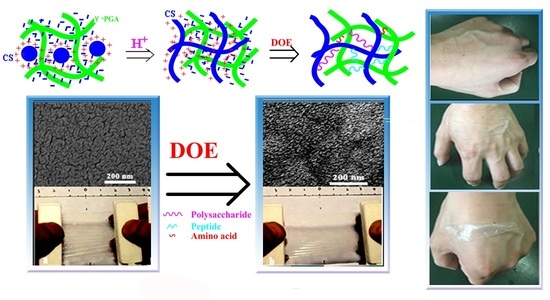
Dendrobium officinale Enzyme Changing the Structure and Behaviors of Chitosan/γ-poly(glutamic acid) Hydrogel for Potential Skin Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of DOE
2.3. Preparation of Precursor Solution of Hydrogel
2.4. Preparation of Hydrogel
2.5. SEM, FTIR, XRD and DTG
2.6. Adsorption of Bovine Serum Albumin
2.7. Water Absorption and Retention
2.8. Antibacterial Behavior
2.9. Tensile Test
2.10. DPPH+ Scavenging Behavior
2.11. MTT Test
2.12. Contact Angle and Rolling Angle
2.13. Air Permeability
2.14. In Vitro Release of DOE
3. Results and Discussion
3.1. Effect of γ-PGA
3.2. Effect of DOE
3.3. Characterization of CS/γ-PGA/DOE Hydrogel for Potential Skin Care
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, C.; Huang, R.; Su, R.; Qi, W.; He, Z. Amphiphilic hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 2899–2910. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B 2018, 6, 510–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Qi, C.; Ma, M.G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Zehnder, A.T.; Zhao, J.; Narita, T.; Creton, C.; Hui, C.-Y. Fracture mechanics of a self-healing hydrogel with covalent and physical crosslinks: A numerical study. J. Mech. Phys. Solids 2018, 120, 79–95. [Google Scholar] [CrossRef] [Green Version]
- Lu Ca Ntonio, A.; Noselli, G.; Trepat, X.; Desimone, A.; Arroyo, M. Hydraulic Fracture and Toughening of a Brittle Layer Bonded to a Hydrogel. Phys. Rev. Lett. 2015, 115, 188105. [Google Scholar] [CrossRef] [Green Version]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Xue, B.; Meng, Q.; Wang, T.; Wu, J.; Luo, D.; Jiang, Q.; Ying, L.; Yi, C.; Wei, W. Electrically Controllable Actuators Based on Supramolecular Peptide Hydrogels. Adv. Funct. Mater. 2016, 26, 9053–9062. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, T.; Wang, X.; Yu, B.; Zhou, F. Freezing Molecular Orientation under Stretch for High Mechanical Strength but Anisotropic Hydrogels. Small 2016, 12, 4386–4392. [Google Scholar] [CrossRef]
- Gong, J. Why are double network hydrogels so tough. Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Yuan, T.; Cui, X.; Liu, X.; Qu, X.; Sun, J. Highly Tough, Stretchable, Self-Healing, and Recyclable Hydrogels Reinforced by in Situ-Formed Polyelectrolyte Complex Nanoparticles. Macromolecules 2019, 52, 3141–3149. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.; Shibayama, M. Structure Characterization of Tetra-PEG Gel by Small-Angle Neutron Scattering. Macromolecules 2009, 42, 1344–1351. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Yang, K.; Lu, T.; Zhan, L.; Zhou, C.; Zhang, N.; Lei, S.; Wang, Y.; Yang, J.; Yan, M.; Lv, G.; et al. Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. Int. J. Biol. Macromol. 2020, 149, 320–330. [Google Scholar] [CrossRef]
- Zhang, H.; Tsang, S.W.; Zhu, Y. Skin-Protection Composition Containing Dendrobium-Based Ingredients. U.S. Patent No. 20170057906A1, 2 March 2017. [Google Scholar]
- Shu, X. Study on Preparation Process and Functional Activity of Dendrobium Jaosu. Master’ Thesis, Anhui Polytechnic University, Wuhu, China, 2019. [Google Scholar]
- Wu, C.H.; Wu, T.T.; Fang, Z.X.; Zheng, J.W.; Xu, S.; Chen, S.G.; Hu, Y.Q.; Ye, X.Q. Formation, characterization and release kinetics of chitosan/gamma-PGA encapsulated nisin nanoparticles. RSC Adv. 2016, 6, 46686–46695. [Google Scholar] [CrossRef]
- Wang, M.; Zang, Y.; Hong, K.; Zhao, X.; Yu, C.; Liu, D.; An, Z.; Wang, L.; Yue, W.; Nie, G. Preparation of pH-sensitive carboxymethyl cellulose/chitosan/alginate hydrogel beads with reticulated shell structure to deliver Bacillus subtilis natto. Int. J. Biol. Macromol. 2021, 192, 684–691. [Google Scholar] [CrossRef]
- Lu, Z.; Yue, S.; Bing, L.; Ying, Z.; Yu, L.F.; Ping, N.C. Preparation and preliminary bioactivity determination of Dendrobium offcinale Kimura et Migo enzyme. Int. J. Food Sci. Technol. 2018, 43, 129–134. [Google Scholar]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Ruggiero, C.; Pastorino, L. Chitosan/dextran multilayer microcapsules for polyphenol co-delivery. Mater. Sci. Eng. C-Mater. 2015, 46, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Li, Q.Q.; Wang, D.; Chen, J.; Huang, J.H.; Zeng, Q.H. The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1. Tradit. Med. Res. 2018, 3, 131–139. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Pawakongbun, T.; Lourith, N. Dendrobium orchid polysaccharide extract: Preparation, characterization and in vivo skin hydrating efficacy. Chin. Herb. Med. 2019, 11, 400–405. [Google Scholar] [CrossRef]
- Nie, G.; Hong, K.; Zhang, E.; Liu, N.; Wang, M.; Wang, L.; Zang, Y. Fabrication of a porous chitosan/poly-(γ-glutamic acid) hydrogel with a high absorption capacity by electrostatic contacts. Int. J. Biol. Macromol. 2020, 159, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Lee, Y.R.; Kang, T.H.; Choi, J.W.; Kimura, S.; Wada, M. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydr. Polym. 2017, 163, 34–42. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.L.; Tang, Z.H.; Zhang, X.F.; Zhong, Y.H.; Yao, S.Z.; Wang, L.S.; Lin, C.W.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Yue, Z.; Che, Y.; Jin, Z.; Wang, S.; Ma, Q.; Zhang, Q.; Tan, Y.; Meng, F. A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly(ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers. J. Biomater. Sci. Polym. Edit. 2019, 30, 1375–1398. [Google Scholar] [CrossRef]
- Ragan, M.A.; Craigie, J.S. Quantitative studies on brown algal phenols IV. Ultraviolet spectrophotometry of extracted polyphenols and implications for measuring dissolved organic matter in sea water. J. Exp. Mar. Biol. Ecol. 1980, 46, 231–239. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, M.J. Removal of heavy metal ions by poly(vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Zhao, J.; Feng, H.; Li, P.; Tong, Z.; Yang, Z.; Li, S.; Yang, J.; Jin, S. Preparation of the chitosan/poly (glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.J.; Zhang, Y.; Yan, S.F.; Liu, Z.W.; He, S.M.; Cui, L.; Yin, J.B. Poly (L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Amosa, M.K.; Jami, M.S.; Ma’an, F. Electrostatic biosorption of COD, Mn and H2S on EFB-based activated carbon produced through steam pyrolysis: An analysis based on surface chemistry, equilibria and kinetics. Waste Biomass Valorization 2016, 7, 109–124. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.; Huo, P. Preparation, characterization, and antimicrobial activity of poly (γ-glutamic acid)/chitosan blends. Polym. Bull. 2019, 76, 2163–2178. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodriguez, M.S.; Debbaudt, A.; Zuniga, A.E.; Casalongue, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Ijadi Bajestani, M.; Mousavi, S.M.; Mousavi, S.B.; Jafari, A.; Shojaosadati, S.A. Purification of extra cellular poly-γ-glutamic acid as an antibacterial agent using anion exchange chromatography. Int. J. Biol. Macromol. 2018, 113, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Han, J.L.; Hsieh, K.H. Antibacterial activity and biocompatibility of a chitosan–γ-poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohydr. Res. 2010, 345, 1774–1780. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.S.; Park, S.H.; Lee, Y.G.; Son, T.I. Polyelectrolyte complex hydrogel composed of chitosan and poly(γ-glutamic acid) for biological application: Preparation, physical properties, and cytocompatibility. J. Appl. Polym. Sci. 2007, 103, 386–394. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Gray, K.M.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Wang, Q.; Payne, G.F. Electrodeposition of a weak polyelectrolyte hydrogel: Remarkable effects of salt on kinetics, structure and properties. Soft Matter 2013, 9, 2703. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. Lwt-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Xu, H.; Matysiak, S. Effect of pH on chitosan hydrogel polymer network structure. Chem. Commun. 2017, 53, 7373–7376. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, A. Amino acid based smart hydrogel: Formation, characterization and fluorescence properties of silver nanoclusters within the hydrogel matrix. Soft Matter 2011, 7, 5300–5308. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Bawa, A.S.; Siddaramaiah. Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhong, T.; Chen, P.; Fu, J.; Jin, Y.; Liu, Y.; Huang, R.; Tan, L. Preparation, characterization and in vitro release study of drug-loaded sodium carboxy-methylcellulose/chitosan composite sponge. PLoS ONE 2018, 13, e0206275. [Google Scholar] [CrossRef] [PubMed]
- Ikhuoria, E.U.; Omorogbe, S.O.; Agbonlahor, O.G.; Iyare, N.O.; Pillai, S.; Aigbodion, A.I. Spectral analysis of the chemical structure of carboxymethylated cellulose produced by green synthesis from coir fibre. Ciênc. Tecnol. Mater. 2017, 29, 55–62. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Mikula, K.; Kossińska, N.; Widera, B.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. Biodegradable hydrogel materials for water storage in agriculture-review of recent research. Desalin. Water Treat. 2020, 194, 324–332. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Cao, Z.; Gao, C.; Duan, H.; Xu, X.; Sun, L.; Gao, N.; Feng, C.; Liu, M. Self-reinforcing injectable hydrogel with both high water content and mechanical strength for bone repair. Chem. Eng. J. 2016, 288, 546–556. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, H.; Li, C.; Lin, L. A novel polyethylene oxide/Dendrobium officinale nanofiber: Preparation, characterization and application in pork packaging. Food Packag. Shelf 2019, 21, 100329. [Google Scholar] [CrossRef]
- Li, B.; Wei, J.; Wei, X.; Tang, K.; Liang, Y.; Shu, K.; Wang, B. Effect of sound wave stress on antioxidant enzyme activities and lipid peroxidation of Dendrobium candidum. Colloids Surface B 2008, 63, 269–275. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Evaluation of chitosan/γ-poly(glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr. Polym. 2011, 84, 812–819. [Google Scholar] [CrossRef]
- Liang, J.; Chen, S.; Hu, Y.; Yang, Y.; Yuan, J.; Wu, Y.; Li, S.; Lin, J.; He, L.; Hou, S.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107, 2201–2210. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Chen, K.; Xiao, C.; Fan, L.; Zhang, B.; Ren, J.; Fang, B. An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. J. Funct. Foods 2019, 53, 44–53. [Google Scholar] [CrossRef]
- Surber, C.; Kottner, J. Skin care products: What do they promise, what do they deliver. J. Tissue Viability 2017, 26, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Hasanuzzaman, M.; Gulshan, F.; Rashid, A. Surface, Mechanical and Shape Memory Properties of Biodegradable Polymers and Their Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Yang, H.; Lin, B.; Han, X.; Chen, P. Study on the influence of crosslinking density and free polysiloxan chain length on oxygen permeability and hydrophilicity of multicomponent silicone hydrogels. Colloid Polym. Sci. 2021, 299, 1327–1335. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [Green Version]






| Sample (Unit) | DOE (±SD) |
|---|---|
| Total sugars (mg/mL) | 131 (±6.2) |
| Total phenols (mg/mL) | 0.186 (±0.04) |
| Total amino acids/peptides (mg/mL) | 1.22 (±0.078) |
| SOD (U/L) | 4750 (±148) |
| Protease (U/mL) | 755 (±9.8) |
| Amylase (U/L) | 330 (±1.5) |
| DPPH+ scavenging activity (%) | 64 (±1.6) |
| Reducing power (OD700) | 1.14 (±0.037) |
| Hydroxyl radical scavenging rate (%) | 30 (±1.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, E.; Yu, C.; Liu, D.; Zhao, S.; Xu, M.; Zhao, X.; Yue, W.; Nie, G. Dendrobium officinale Enzyme Changing the Structure and Behaviors of Chitosan/γ-poly(glutamic acid) Hydrogel for Potential Skin Care. Polymers 2022, 14, 2070. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102070
Wang M, Zhang E, Yu C, Liu D, Zhao S, Xu M, Zhao X, Yue W, Nie G. Dendrobium officinale Enzyme Changing the Structure and Behaviors of Chitosan/γ-poly(glutamic acid) Hydrogel for Potential Skin Care. Polymers. 2022; 14(10):2070. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102070
Chicago/Turabian StyleWang, Mengmeng, Erwei Zhang, Chenrui Yu, Dandan Liu, Shiguang Zhao, Maodong Xu, Xiaofeng Zhao, Wenjin Yue, and Guangjun Nie. 2022. "Dendrobium officinale Enzyme Changing the Structure and Behaviors of Chitosan/γ-poly(glutamic acid) Hydrogel for Potential Skin Care" Polymers 14, no. 10: 2070. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102070