Biocompatibility and Hemolytic Activity Studies of Synthesized Alginate-Based Polyurethanes
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals
Alginate (ALG ≥ 99.0%)-Preliminary Information
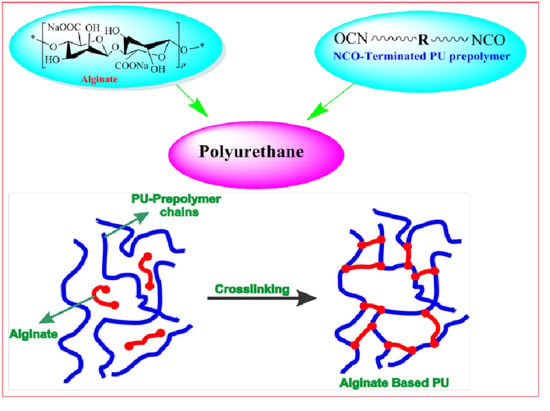
2.2. Synthesis of PU Materials
2.3. Measurements
3. Results and Discussion
3.1. FTIR Analysis
3.2. 1H NMR Studies of PUs
3.3. XRD Analysis of Prepared Composites
3.4. TGA Analysis
3.5. DSC Analysis of PUs
3.6. Evaluation of Hemolytic Activity
3.7. Tensile Strengths of Biodegradable Composites
3.8. Contact Angle Measurements
4. Comparative Profile
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, A.A.; Safian, M.T.U.; Rashid, M.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Chapter One-Introduction of Smart Polymer Nanocomposites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 1–25. [Google Scholar]
- Feng, G.; Ma, Y.; Zhang, M.; Jia, P.; Liu, C.; Zhou, Y. Synthesis of Bio-base Plasticizer Using Waste Cooking Oil and Its Performance Testing in Soft Poly(vinyl chloride) Films. J. Bioresour. Bioprod. 2019, 4, 99–110. [Google Scholar] [CrossRef]
- Zhou, B.; Hu, Y.; Li, J.; Li, B. Chitosan/phosvitin antibacterial films fabricated via layer-by-layer deposition. Int. J. Biol. Macromol. 2014, 64, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Zia, K.M.; Zafar, K.; Khosa, M.K.; Akram, N.; Ajmal, M.; Imran, M.; Iqbal, M.N. Synthesis and molecular characterization of chitosan/starch blends based polyurethanes. Int. J. Biol. Macromol. 2020, 146, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.-Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Hu, Q.; Bae, M.; Fleming, E.; Lee, J.-Y.; Luo, Y. Biocompatible polymeric nanoparticles with exceptional gastrointestinal stability as oral delivery vehicles for lipophilic bioactives. Food Hydrocoll. 2019, 89, 386–395. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Long, X.; Lin, W.; Du, B.; Yin, H.; Lan, W.; Zhao, D.; Li, Z.; Li, J.; Luo, F.; et al. Bioactive 3D porous cobalt-doped alginate/waterborne polyurethane scaffolds with a coral reef-like rough surface for nerve tissue engineering application. J. Mater. Chem. B 2021, 9, 322–335. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.; Zhang, Y.; Liang, H.; Liang, D.; Jiang, J.; Lu, Q.; Quirino, R.L.; Zhang, C. Thermosetting polyurethanes prepared with the aid of a fully bio-based emulsifier with high bio-content, high solid content, and superior mechanical properties. Green Chem. 2018, 21, 526–537. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Iqbal, S.; Rahman, M.S.U.; Ahmed, S.; Shabir, G.; Javaid, M.A. Relationship of hard segment concentration in polyurethane-urea elastomers with mechanical, thermal and drug release properties. J. Drug Deliv. Sci. Technol. 2017, 37, 88–96. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Kim, Y. The Korean Wave: Korean Media Go Global, 1st ed.; Routledge: London, UK, 2013; p. 250. [Google Scholar] [CrossRef]
- Montaser, A.S.; Jlassi, K.; Ramadan, M.A.; Sleem, A.A.; Attia, M.F. Alginate, gelatin, and carboxymethyl cellulose coated nonwoven fabrics containing antimicrobial AgNPs for skin wound healing in rats. Int. J. Biol. Macromol. 2021, 173, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Imran, M.; Lian, Q.; Shehzad, F.K.; Athir, N.; Zhang, J.; Cheng, J. Curcumin incorporated polyurethane urea elastomers with tunable thermo-mechanical properties. React. Funct. Polym. 2018, 128, 97–103. [Google Scholar] [CrossRef]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Javaid, M.A.; Rizwan, M.; Khera, R.A.; Zia, K.M.; Saito, K.; Zuber, M.; Iqbal, J.; Langer, P. Thermal degradation behavior and X-ray diffraction studies of chitosan based polyurethane bio-nanocomposites using different diisocyanates. Int. J. Biol. Macromol. 2018, 117, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, J.D. In vitro hemolysis of human erythrocytes—By plant extracts with antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 239–243. [Google Scholar] [CrossRef]
- Safian, M.T.; Umar, K.; Parveen, T.; Yaqoob, A.A.; Ibrahim, M.N.M. Chapter Eight-Biomedical applications of smart polymer composites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Woodhead Publishing Series in Composites Science and Engineering; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 183–204. [Google Scholar]
- Zia, K.M.; Bhatti, I.A.; Barikani, M.; Zuber, M.; Bhatti, H.N. XRD studies of polyurethane elastomers based on chitin/1,4-butane diol blends. Carbohydr. Polym. 2009, 76, 183–187. [Google Scholar] [CrossRef]
- Tabasum, S.; Zuber, M.; Jabbar, A.; Zia, K.M. Properties of the modified cellulosic fabrics using polyurethane acrylate copolymers. Carbohydr. Polym. 2013, 94, 866–873. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Virgili, J.M.; Larive, M.Z.; Wendt, B.L. Synthesis of aniline-terminated polyethers and resulting polyurethane/polyurea elastomers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1730–1742. [Google Scholar] [CrossRef]
- Fiayaz, M.; Zia, K.M.; Javaid, M.A.; Rehman, S.; Chatha, S.A.S.; Zuber, M. Synthesis and characterization of hydroxyethyl cellulose (HEC)-TiO2-based polyurethane bionanocomposites. Korean J. Chem. Eng. 2020, 37, 2351–2358. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. A simple approach for morphology tailoring of alginate particles by manipulation ionic nature of polyurethanes. Int. J. Biol. Macromol. 2014, 66, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Javaid, M.A.; Younas, M.; Zafar, I.; Khera, R.A.; Zia, K.M.; Jabeen, S. Mathematical modeling and experimental study of mechanical properties of chitosan based polyurethanes: Effect of diisocyanate nature by mixture design approach. Int. J. Biol. Macromol. 2019, 124, 321–330. [Google Scholar] [CrossRef]
- Javaid, M.A.; Zia, K.M.; Ilyas, H.N.; Sidra; Yaqub, N.; Bhatti, I.A.; Rehan, M.; Shoaib, M.; Bahadur, A. Influence of chitosan/1,4-butanediol blends on the thermal and surface behavior of polycaprolactone diol-based polyurethanes. Int. J. Biol. Macromol. 2019, 141, 1022–1034. [Google Scholar] [CrossRef]
- Javaid, M.A.; Zia, K.M.; Khera, R.A.; Jabeen, S.; Mumtaz, I.; Younis, M.A.; Shoaib, M.; Bhatti, I.A. Evaluation of cytotoxicity, hemocompatibility and spectral studies of chitosan assisted polyurethanes prepared with various diisocyanates. Int. J. Biol. Macromol. 2019, 129, 116–126. [Google Scholar] [CrossRef]
- Javaid, M.A.; Khera, R.A.; Zia, K.M.; Saito, K.; Bhatti, I.A.; Asghar, M. Synthesis and characterization of chitosan modified polyurethane bio-nanocomposites with biomedical potential. Int. J. Biol. Macromol. 2018, 115, 375–384. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Rapid determination of alginate monomer composition using Raman spectroscopy and chemometrics. Gums Stabilisers Food Ind. 2008, 14, 543–551. [Google Scholar]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef] [Green Version]
- Zia, K.M.; Mahmood, K.; Zuber, M.; Jamil, T.; Shafiq, M. Chitin based polyurethanes using hydroxyl terminated polybutadiene. Part I: Molecular engineering. Int. J. Biol. Macromol. 2013, 59, 320–327. [Google Scholar] [CrossRef]
- Hassan, M.M.; Aly, R.O.; El-Ghandour, A.; Abdelnaby, H.A. Effect of gamma irradiation on some properties of reclaimed rubber/nitrile–butadiene rubber blend and its swelling in motor and brake oils. J. Elastomers Plast. 2012, 45, 77–94. [Google Scholar] [CrossRef]
- Joo, Y.-S.; Cha, J.-R.; Gong, M.-S. Biodegradable shape-memory polymers using polycaprolactone and isosorbide based polyurethane blends. Mater. Sci. Eng. C 2018, 91, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]








| Sr. No. | Sample Code | HTPB a (Moles) | HMDI b (Moles) | 1,4-BDO c (Moles) | Alginate |
|---|---|---|---|---|---|
| 1 | PUK-1 | 1 | 3 | 2 | 0 |
| 2 | PUK-2 | 1 | 3 | 1.5 | 0.5 |
| 3 | PUK-3 | 1 | 3 | 1 | 1 |
| 4 | PUK-4 | 1 | 3 | 0.5 | 1.5 |
| 5 | PUK-5 | 1 | 3 | 0 | 2 |
| Sr. No. | Sample Code | Chain Extender Ratio Alginate:BDO | 2Ɵ (deg) | d-Spacing (Å) | Intensity (a.u) | Glass Transition |
|---|---|---|---|---|---|---|
| 1 | PUK-1 | 0.0:2.0 | 25.0 | 3.56 | 20,197 | 40.27 |
| 2 | PUK-2 | 0.50:1.50 | 25.1 | 3.55 | 20,641 | 40.79 |
| 3 | PUK-3 | 1.00:1.00 | 25.2 | 3.54 | 22,857 | 41.59 |
| 4 | PUK-4 | 1.50:0.50 | 25.3 | 3.53 | 23,606 | 42.69 |
| 5 | PUK-5 | 2.0:0.0 | 25.4 | 3.51 | 32,347 | 45.35 |
| Sr. | Sample | Tonset a | Ti b | T10 c | T20 c | T50 c | T60 c | T80 c | Residue d |
|---|---|---|---|---|---|---|---|---|---|
| # | Code | (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | (%) | (%) |
| 1 | PUK-1 | 30 | 260 | 343 | 368 | 393 | 419 | 436 | 1.01 |
| 2 | PUK-2 | 29 | 270 | 356 | 379 | 400 | 422 | 440 | 3.30 |
| 3 | PUK-3 | 28 | 275 | 360 | 384 | 405 | 429 | 448 | 5.00 |
| 4 | PUK-4 | 28 | 273 | 363 | 387 | 408 | 431 | 453 | 6.01 |
| 5 | PUK-5 | 29 | 254 | 364 | 363 | 387 | 408 | 455 | 8.01 |
| Sample Code | % Hemolysis a (Mean) | Standard Deviation |
|---|---|---|
| PUK-1 | 13.9 | 0.24 |
| PUK-2 | 7.72 | 0.34 |
| PUK-3 | 6.5 | 0.15 |
| PUK-4 | 5.4 | 0.68 |
| PUK-5 | 3.99 | 0.41 |
| DMF b | 0.11 | 0.03 |
| PBS c | 0.06 | 0.03 |
| Triton-X-100 | 95.1 | 0.52 |
| Sr. No. | Sample Code | Tensile Strength (M Pa) | Elongation at Break (%) | Hardness (Shore A) |
|---|---|---|---|---|
| 1 | PUK-1 | 7.23 ± 1.05 | 204 ± 0.85 | 87.32 ± 0.89 |
| 2 | PUK-2 | 8.52 ± 1.21 | 156 ± 0.89 | 89.52 ± 1.85 |
| 3 | PUK-3 | 9.34 ± 1.41 | 138 ± 1.05 | 90.41 ± 0.87 |
| 4 | PUK-4 | 9.67 ± 1.01 | 120 ± 1.15 | 92.30 ± 1.99 |
| 5 | PUK-5 | 9.8 ± 1.55 | 113 ± 1.03 | 93.12 ± 1.37 |
| Sample | Contact Angle (θ°) | Water Absorption * (%) |
|---|---|---|
| PUK-1 | 74.13 ± 2.4 | 10.69 ± 2.72 |
| PUK-2 | 72.24 ± 3.11 | 10. 92 ± 3.41 |
| PUK-3 | 71.24 ± 1.85 | 11.59 ± 2.52 |
| PUK-4 | 70.32 ± 2.63 | 13.66 ± 3.43 |
| PUK-5 | 70.10 ± 2.31 | 14.78 ± 2.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, K.; Zia, K.M.; Alzhrani, R.M.; Almalki, A.H.; Alshehri, S. Biocompatibility and Hemolytic Activity Studies of Synthesized Alginate-Based Polyurethanes. Polymers 2022, 14, 2091. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102091
Zafar K, Zia KM, Alzhrani RM, Almalki AH, Alshehri S. Biocompatibility and Hemolytic Activity Studies of Synthesized Alginate-Based Polyurethanes. Polymers. 2022; 14(10):2091. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102091
Chicago/Turabian StyleZafar, Kashif, Khalid Mahmood Zia, Rami M. Alzhrani, Atiah H. Almalki, and Sameer Alshehri. 2022. "Biocompatibility and Hemolytic Activity Studies of Synthesized Alginate-Based Polyurethanes" Polymers 14, no. 10: 2091. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14102091