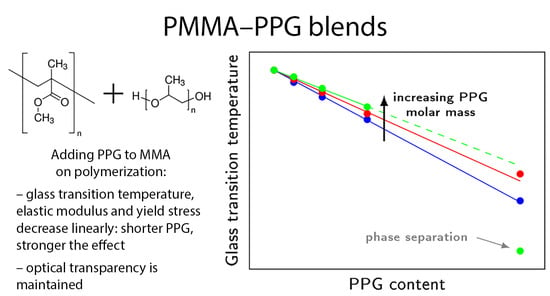
Transparent Polymer Blends of Poly(methyl methacrylate) and Poly(propylene glycol)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. X-ray Diffraction
2.4. Dynamic Mechanical Thermal Analysis
2.5. Compression Testing
3. Results
3.1. Synthesis
3.2. X-ray Diffraction
3.3. Dynamic Mechanical Thermal Analysis
3.4. Compression Testing
4. Discussion
4.1. X-ray Diffraction
4.2. Dynamic Mechanical Thermal Analysis
4.3. Compression Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMMA | Poly(methyl methacrylate) |
| PPG | Poly(propylene glycol) |
| PG | Propylene glycol |
| MW | Molecular weight |
| XRD | X-ray diffraction |
| DMTA | Dynamic mechanical thermal analysis |
References
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Pepose, J.S.; Burke, J.; Qazi, M.A. Benefits and barriers of accommodating intraocular lenses. Curr. Opin. Ophthalmol. 2017, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á. Latest Improvements of Acrylic-Based Polymer Properties for Biomedical Applications. In Acrylic Polymers in Healthcare; Reddy, B.S., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S. Acrylates in Dental Applications. In Acrylic Polymers in Healthcare; Reddy, B.S., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Abaci, U.; Guney, H.Y.; Yilmazoglu, M. Plasticizer effect on dielectric properties of poly(methyl methacrylate)/titanium dioxide composites. Polym. Polym. Compos. 2021, 29, S565–S574. [Google Scholar] [CrossRef]
- Liberman, S.A.; Gomes, A.D.S.; Macchi, E.M. Compatibility in poly(ethylene oxide)–poly(methyl methacrylate) blends. J. Polym. Sci. Polym. Chem. Ed. 2018, 22, 2809–2815. [Google Scholar] [CrossRef]
- Feinberg, E.C.; Hernandez, H.L.; Plantz, C.L.; Mejia, E.B.; Sottos, N.R.; White, S.R.; Moore, J.S. Cyclic Poly(phthalaldehyde): Thermoforming a Bulk Transient Material. ACS Macro Lett. 2018, 7, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Olayemi, J.Y.; Oniyangi, N.A. Three-stage interaction of dimethyl phthalate, dibutyl phthalate, and poly(vinyl acetate) with poly(methyl methacrylate). J. Appl. Polym. Sci. 2018, 26, 4059–4067. [Google Scholar] [CrossRef]
- Scott, M.P.; Brazel, C.S.; Benton, M.G.; Mays, J.W.; Holbrey, J.D.; Rogers, R.D. Application of ionic liquids as plasticizers for poly(methyl methacrylate). Chem. Commun. 2002, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Babo, S.; Ferreira, J.L.; Ramos, A.M.; Micheluz, A.; Pamplona, M.; Casimiro, M.H.; Ferreira, L.M.; Melo, M.J. Characterization and Long-Term Stability of Historical PMMA: Impact of Additives and Acrylic Sheet Industrial Production Processes. Polymers 2020, 12, 2198. [Google Scholar] [CrossRef]
- Stark, T.D.; Choi, H.; Diebel, P.W. Influence of plasticizer molecular weight on plasticizer retention in PVC geomembranes. Geosynth. Int. 2005, 12, 99–110. [Google Scholar] [CrossRef]
- England, D.; Slater, L.; Mourey, T.; Texter, J. Thermomechanical Synergisms from Ionic Liquid Doping of Poly(methyl methacrylate). ACS Macro Lett. 2013, 2, 901–905. [Google Scholar] [CrossRef]
- Agrawal, P.; Parmar, P.; Mallick, R.; Bajpai, R. Study of surface morphology and characterization of polymethyl methacrylate:polychlorotrifluoro ethyelene polyblend using SEM, DSC and X-ray diffraction techniques. Indian J. Pure Appl. Phys. 2008, 45, 193–197. [Google Scholar]
- Storey, R.F.; Mauritz, K.A.; Cox, B.D. Diffusion of various dialkyl phthalate plasticizers in PVC. Macromolecules 1989, 22, 289–294. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Sarazin, P.; Favis, B.D. High molecular weight plasticizers in thermoplastic starch/polyethylene blends. J. Mater. Sci. 2013, 48, 1799–1811. [Google Scholar] [CrossRef]
- Burgos, N.; Tolaguera, D.; Fiori, S.; Jiménez, A. Synthesis and Characterization of Lactic Acid Oligomers: Evaluation of Performance as Poly(Lactic Acid) Plasticizers. J. Polym. Environ. 2014, 22, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, N.; Wesslén, B. Tributyl citrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Yin, B.; Hakkarainen, M. Oligomeric isosorbide esters as alternative renewable resource plasticizers for PVC. J. Appl. Polym. Sci. 2011, 119, 2400–2407. [Google Scholar] [CrossRef]
- IHS Markit. Chemical Economics Handbook: Plasticizers; SRI International: Palo Alto, CA, USA, 2018. [Google Scholar]
- Guan, S.; Wang, W.; Zheng, J.; Xu, C. A method to achieve full incorporation of PMMA-based gel electrolyte in fiber-structured PVB for solid-state electrochromic device fabrication. Electrochim. Acta 2020, 354, 136702. [Google Scholar] [CrossRef]
- Jain, S.; Bajpai, A. Designing polyethylene glycol (PEG) – plasticized membranes of poly(vinyl alcohol-g-methyl methacrylate) and investigation of water sorption and blood compatibility behaviors. Des. Monomers Polym. 2013, 16, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Dhatarwal, P.; Sengwa, R.J. Effects of PEG plasticizer concentrations and film preparation methods on the structural, dielectric and electrical properties of PEO–PMMA blend based plasticized solid polymer electrolyte films. Indian J. Pure Appl. Phys. 2017, 55, 7–18. [Google Scholar]
- Philip, P.; Tomlal Jose, E.; Chacko, J.K.; Philip, K.C.; Thomas, P.C. Preparation and characterisation of surface roughened PMMA electrospun nanofibers from PEO–PMMA polymer blend nanofibers. Polym. Test. 2019, 74, 257–265. [Google Scholar] [CrossRef]
- Philip, P.; Jose, T.; Vanchippurackal, I.V. Structurally modified electrospun poly(methyl methacrylate) nanofibers as advanced host matrices for PbS quantum dots. J. Appl. Polym. Sci. 2021, 138, 50534. [Google Scholar] [CrossRef]
- Carvalho, L.D.d.; Peres, B.U.; Maezono, H.; Shen, Y.; Haapasalo, M.; Jackson, J.; Carvalho, R.M.; Manso, A.P. Doxycycline release and antibacterial activity from PMMA/PEO electrospun fiber mats. J. Appl. Oral Sci. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.; Huang, C.; Liu, G. Crystallization of Poly (ethylene glycol) in Poly (methyl methacrylate) Networks. Mater. Sci. 2013, 19, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Fowles, J.R.; Banton, M.I.; Pottenger, L.H. A toxicological review of the propylene glycols. Crit. Rev. Toxicol. 2013, 43, 363–390. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- Shobhana, E. X-Ray Diffraction and UV-Visible Studies of PMMA Thin Films. Int. J. Mod. Eng. Res. 2012, 2, 1092–1095. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korigodskii, A.A.; Zhirnov, A.E.; Kechekyan, A.S.; Zezin, S.B. Transparent Polymer Blends of Poly(methyl methacrylate) and Poly(propylene glycol). Polymers 2022, 14, 2171. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112171
Korigodskii AA, Zhirnov AE, Kechekyan AS, Zezin SB. Transparent Polymer Blends of Poly(methyl methacrylate) and Poly(propylene glycol). Polymers. 2022; 14(11):2171. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112171
Chicago/Turabian StyleKorigodskii, Andrei A., Artem E. Zhirnov, Alexander S. Kechekyan, and Sergey B. Zezin. 2022. "Transparent Polymer Blends of Poly(methyl methacrylate) and Poly(propylene glycol)" Polymers 14, no. 11: 2171. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112171