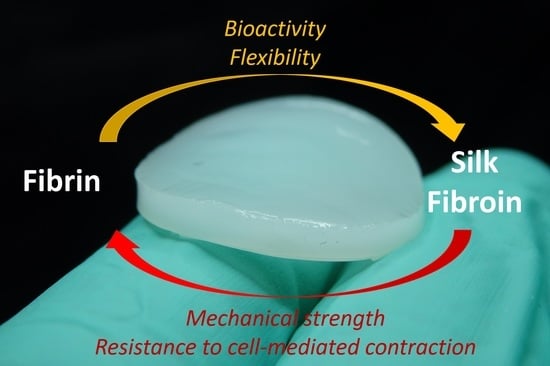
Silk Fibroin as Adjuvant in the Fabrication of Mechanically Stable Fibrin Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibrin and Silk Fibroin Preparation
2.2. Fabrication of Composite Scaffolds
2.3. Scanning Electron Microscopy (SEM)
2.4. Rheological Characterization
2.5. Cell Culture Study
2.5.1. Culture of Human Umbilical Vein Endothelial Cells (HUVECs) and Human Umbilical Arterial Smooth Muscle Cells (HUASMCs)
2.5.2. Seeding of HUVECs and Evaluation of Cell Adhesion
2.5.3. Seeding of HUASMCs and Evaluation of Scaffold Contraction
2.5.4. Fixation and Staining of the Cell-Seeded Scaffolds
2.5.5. Confocal Microscopy Evaluation
2.6. Fabrication of Tubular Scaffolds
2.7. Burst Strength
2.8. Statistical Analysis
3. Results
3.1. Fabrication of the Scaffolds
3.2. Rheological Characterization
3.3. Cell Adhesion of HUVECs to the Composite Scaffolds
3.4. Evaluation of the Cell-Mediated Scaffold Contraction
3.5. Fabrication of Composite Scaffolds with Tubular Shape
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gore, P.M.; Kandasubramanian, B. Functionalized Aramid Fibers and Composites for Protective Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 16537–16563. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Taylor, P.M.; Cass, A.E.G.; Yacoub, M.H. Extracellular matrix scaffolds for tissue engineering heart valves. Prog. Pediatric Cardiol. 2006, 21, 219–225. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Cho, N.-J. Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2014, 7, 53–63. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.; Nürnberger, S.; Redl, H. Fibrin: The Very First Biomimetic Glue—Still a Great Tool. In Biological Adhesive Systems: From Nature to Technical and Medical Application; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria, 2010; pp. 225–236. [Google Scholar]
- Spicer, P.P.; Mikos, A.G. Fibrin glue as a drug delivery system. J. Control. Release J. Control. Release Soc. 2010, 148, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Persinal-Medina, M.; Llames, S.; Chacón, M.; Vázquez, N.; Pevida, M.; Alcalde, I.; Alonso-Alonso, S.; Martínez-López, L.M.; Merayo-Lloves, J.; Meana, Á. Polymerizable Skin Hydrogel for Full Thickness Wound Healing. Int. J. Mol. Sci. 2022, 23, 4837. [Google Scholar] [CrossRef]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Lopezcarasa-Hernandez, G.; Perez-Vazquez, J.F.; Guerrero-Naranjo, J.L.; Martinez-Castellanos, M.A. Versatility of use of fibrin glue in wound closure and vitreo-retinal surgery. Int. J. Retin. Vitr. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Gauthier, O.; Masson, M.; Malard, O.; Moreau, A.; Fellah, B.H.; Bilban, M.; Spaethe, R.; Daculsi, G.; Guicheux, J. Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2009, 89, 176–185. [Google Scholar] [CrossRef]
- Patel, S.; Rodriguez-Merchan, E.C.; Haddad, F.S. The use of fibrin glue in surgery of the knee. J. Bone Jt. Surgery. Br. Vol. 2010, 92-B, 1325–1331. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.; Neusser, C.; Kruse, M.; Mulderrig, S.; Wolf, F.; Spillner, J.; Schmitz-Rode, T.; Jockenhoevel, S.; Mela, P. Tissue-Engineered Fibrin-Based Heart Valve with Bio-Inspired Textile Reinforcement. Adv. Healthc. Mater. 2016, 5, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.; Paefgen, V.; Winz, O.; Mertens, M.; Koch, S.; Gross-Weege, N.; Morgenroth, A.; Rix, A.; Schnoering, H.; Chalabi, K.; et al. MR and PET-CT monitoring of tissue-engineered vascular grafts in the ovine carotid artery. Biomaterials 2019, 216, 119228. [Google Scholar] [CrossRef]
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.A.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. J. Eur. Assoc. Cardio-Thorac. Surg. 2000, 17, 587–591. [Google Scholar] [CrossRef]
- Rosso, M.P.O.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.; Ferreira Júnior, R.S.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.B.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190038. [Google Scholar] [CrossRef]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Schmoekel, H.G.; Weber, F.E.; Schense, J.C.; Grätz, K.W.; Schawalder, P.; Hubbell, J.A. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 2005, 89, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, M.C.; Felice, F.; Balbarini, A.; Di Stefano, R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 301–310. [Google Scholar] [CrossRef]
- Thompson, W.D.; Smith, E.B.; Stirk, C.M.; Marshall, F.I.; Stout, A.J.; Kocchar, A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J. Pathol. 1992, 168, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Robson, S.C.; Shephard, E.G.; Kirsch, R.E. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br. J. Haematol. 1994, 86, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel—Advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. J. Eur. Assoc. Cardio-Thorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Samal, J.; Weinandy, S.; Weinandy, A.; Helmedag, M.; Rongen, L.; Hermanns-Sachweh, B.; Kundu, S.C.; Jockenhoevel, S. Co-Culture of Human Endothelial Cells and Foreskin Fibroblasts on 3D Silk-Fibrin Scaffolds Supports Vascularization. Macromol. Biosci. 2015, 15, 1433–1446. [Google Scholar] [CrossRef]
- Goczkowski, M.; Gobin, M.; Hindié, M.; Agniel, R.; Larreta-Garde, V. Properties of interpenetrating polymer networks associating fibrin and silk fibroin networks obtained by a double enzymatic method. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109931. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Malischewski, A.; Moreira, R.; Hurtado, L.; Gesché, V.; Schmitz-Rode, T.; Jockenhoevel, S.; Mela, P. Umbilical cord as human cell source for mitral valve tissue engineering—Venous vs. arterial cells. Biomed. Technik. Biomed. Eng. 2017, 62, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Velz, T.; Alves, N.; Gesche, V.N.; Malischewski, A.; Schmitz-Rode, T.; Frese, J.; Jockenhoevel, S.; Mela, P. Tissue-engineered heart valve with a tubular leaflet design for minimally invasive transcatheter implantation. Tissue Eng. Part C Methods 2015, 21, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.J. Cell Adhesion Assays. In Extracellular Matrix Protocols; Streuli, C.H., Grant, M.E., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 279–285. [Google Scholar]
- Safinsha, S.; Mubarak Ali, M. Composite scaffolds in tissue engineering. Mater. Today Proc. 2020, 24, 2318–2329. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Y.; Powell, H.M.; James Lee, L.; Belury, M.A.; Lannutti, J.J.; Kniss, D.A. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 2007, 28, 450–458. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non-woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2004, 15, 41–57. [Google Scholar] [CrossRef]
- Kwan, H.; Chisari, E.; Khan, W.S. Cell-Free Scaffolds as a Monotherapy for Focal Chondral Knee Defects. Materials 2020, 13, 306. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-J.; Jiang, D.; Zhang, Z.-Z.; Chen, Y.-R.; Yang, Z.-D.; Zhang, J.-Y.; Shi, J.; Wang, X.; Yu, J.-K. Biomimetic Nanosilica–Collagen Scaffolds for In Situ Bone Regeneration: Toward a Cell-Free, One-Step Surgery. Adv. Mater. 2019, 31, 1904341. [Google Scholar] [CrossRef] [PubMed]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A. Biomaterial-driven in situ cardiovascular tissue engineering-a multi-disciplinary perspective. NPJ Regen. Med. 2017, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Sumpio, B.E.; Timothy Riley, J.; Dardik, A. Cells in focus: Endothelial cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef]
- Pang, J.H.; Farhatnia, Y.; Godarzi, F.; Tan, A.; Rajadas, J.; Cousins, B.G.; Seifalian, A.M. In situ Endothelialization: Bioengineering Considerations to Translation. Small 2015, 11, 6248–6264. [Google Scholar] [CrossRef] [PubMed]
- Grasman, J.M.; O’Brien, M.P.; Ackerman, K.; Gagnon, K.A.; Wong, G.M.; Pins, G.D. The Effect of Sterilization Methods on the Structural and Chemical Properties of Fibrin Microthread Scaffolds. Macromol. Biosci. 2016, 16, 836–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, A.; Acosta, S.; Hernández, R.; Elvira, C.; Jorcano, J.L.; Velasco, D. Contraction of fibrin-derived matrices and its implications for in vitro human skin bioengineering. J. Biomed. Mater. Res. Part A 2021, 109, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Stojic, M.; Ródenas-Rochina, J.; López-Donaire, M.L.; González de Torre, I.; González Pérez, M.; Rodríguez-Cabello, J.C.; Vojtová, L.; Jorcano, J.L.; Velasco, D. Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers 2021, 13, 2114. [Google Scholar] [CrossRef]
- He, S.; Fontaine, A.A.; Schwammenthal, E.; Yoganathan, A.P.; Levine, R.A. Integrated mechanism for functional mitral regurgitation: Leaflet restriction versus coapting force: In vitro studies. Circulation 1997, 96, 1826–1834. [Google Scholar] [CrossRef]









| Nomenclature | Ethanol Bath | [Fibrin] | [Silk Fibroin] |
|---|---|---|---|
| Fib5_NT |  | 5 mg/mL | / |
| Fib5 |  | 5 mg/mL | / |
| Fib5-Silk 0.1% |  | 5 mg/mL | 0.1% |
| Fib5-Silk 0.5% |  | 5 mg/mL | 0.5% |
| Fib5-Silk 1% |  | 5 mg/mL | 1% |
| Scaffold | Fib5 | Fib5-Silk 0.1% | Fib5-Silk 0.5% | Fib5-Silk 1% |
|---|---|---|---|---|
| tan δ | 0.078 ± 0.010 | 0.080 ± 0.003 | 0.078 ± 0.003 | 0.074 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Maachi, I.; Kyriakou, S.; Rütten, S.; Kopp, A.; Köpf, M.; Jockenhoevel, S.; Fernández-Colino, A. Silk Fibroin as Adjuvant in the Fabrication of Mechanically Stable Fibrin Biocomposites. Polymers 2022, 14, 2251. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112251
El Maachi I, Kyriakou S, Rütten S, Kopp A, Köpf M, Jockenhoevel S, Fernández-Colino A. Silk Fibroin as Adjuvant in the Fabrication of Mechanically Stable Fibrin Biocomposites. Polymers. 2022; 14(11):2251. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112251
Chicago/Turabian StyleEl Maachi, Ikram, Stavroula Kyriakou, Stephan Rütten, Alexander Kopp, Marius Köpf, Stefan Jockenhoevel, and Alicia Fernández-Colino. 2022. "Silk Fibroin as Adjuvant in the Fabrication of Mechanically Stable Fibrin Biocomposites" Polymers 14, no. 11: 2251. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112251