Viscoelastic and Properties of Amphiphilic Chitin in Plasticised Polylactic Acid/Starch Biocomposite
Abstract
:1. Introduction
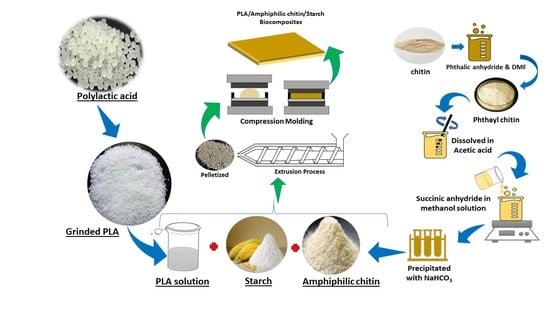
2. Materials and Methods
Materials
3. Results
3.1. Properties of Chitin and Amphiphilic Chitin
3.2. Properties of Neat PLA and Biocomposite
3.3. Thermal Properties of Neat PLA and Biocomposites
3.4. Tensile Properties of Neat PLA and Biocomposite
3.5. Morphological Properties of Neat PLA and Biocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almenar, E.; Auras, R. Permeation, sorption, and diffusion in poly (lactic acid). In Poly (Lactic Acid) Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 155–179. [Google Scholar]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mahmood, S.H.; Pin, J.-M.; Li, R.; Lee, P.C.; Park, C.B. Determination of CO2 solubility in semi-crystalline polylactic acid with consideration of rigid amorphous fraction. Int. J. Biol. Macromol. 2022, 204, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline polysaccharides: A review. Carbohydr. Polym. 2022, 275, 118624. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2021, 26, 126. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno) nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar]
- Rodriguez-Gonzalez, F.; Ramsay, B.; Favis, B. High performance LDPE/thermoplastic starch blends: A sustainable alternative to pure polyethylene. Polymer 2003, 44, 1517–1526. [Google Scholar] [CrossRef]
- Gao, H.; Qiang, T. Fracture surface morphology and impact strength of cellulose/PLA composites. Materials 2017, 10, 624. [Google Scholar] [CrossRef]
- Gadgey, K.K.; Bahekar, A. Studies on extraction methods of chitin from crab shell and investigation of its mechanical properties. Int. J. Mech. Eng. Technol. 2017, 8, 220–231. [Google Scholar]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene oxide filled lignin/starch polymer bionanocomposite: Structural, physical, and mechanical studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Im, S.S.; Kim, S.H.; Kim, Y.H. Biodegradable polymer blends of poly (L-lactic acid) and gelatinized starch. Polym. Eng. Sci. 2000, 40, 2539–2550. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Móczó, J.; Kun, D.; Bódiné Fekete, E. Desiccant effect of starch in polylactic acid composites. Express Polym. Lett. 2018, 12, 1014–1024. [Google Scholar] [CrossRef]
- Wilfred, O.; Tai, H.; Marriott, R.; Liu, Q.; Tverezovskiy, V.; Curling, S.; Tai, H.; Fan, Z.; Wang, W. Biodegradation of polylactic acid and starch composites in compost and soil. Int. J. Nano Res. 2018, 1, 1–11. [Google Scholar]
- Gazzotti, S.; Rampazzo, R.; Hakkarainen, M.; Bussini, D.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Cellulose nanofibrils as reinforcing agents for PLA-based nanocomposites: An in situ approach. Compos. Sci. Technol. 2019, 171, 94–102. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Cao, J.; Wei, S.; Tan, H.; Zhang, Y. Effect of starch/polylactic acid ratio on the interdependence of two-phase and the properties of composites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 1108–1114. [Google Scholar] [CrossRef]
- Ogunrinola, T.; Akpan, U. Production of cassava starch bioplastic film reinforced with poly-lactic acid (PLA). Int. J. Eng. Res. Adv. Technol. 2018, 4, 56–61. [Google Scholar] [CrossRef]
- Aranda-García, F.J.; González-Núñez, R.; Jasso-Gastinel, C.; Mendizábal, E. Water absorption and thermomechanical characterization of extruded starch/poly (lactic acid)/agave bagasse fiber bioplastic composites. Int. J. Polym. Sci. 2015, 2015, 343294. [Google Scholar] [CrossRef] [Green Version]
- Cinelli, P.; Coltelli, M.; Mallegni, N.; Morganti, P.; Lazzeri, A. Degradability and sustainability of nanocomposites based on polylactic acid and chitin nano fibrils. Chem. Eng. Trans. 2017, 60. [Google Scholar] [CrossRef]
- da Mata Cunha, O.; Lima, A.M.F.; Assis, O.B.G.; Tiera, M.J.; de Oliveira Tiera, V.A. Amphiphilic diethylaminoethyl chitosan of high molecular weight as an edible film. Int. J. Biol. Macromol. 2020, 164, 3411–3420. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhuang, Y.; Mu, Q.; Wang, M. Preparation, characterization and aggregation behavior of amphiphilic chitosan derivative having poly (l-lactic acid) side chains. Carbohydr. Polym. 2008, 72, 60–66. [Google Scholar] [CrossRef]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: Facile preparation of a precursor useful for chemical modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.; Stepnova, E.; Babak, V.; Krayukhina, M.; Berezin, B.; Yamskov, I. Amphiphilic N-[2 (3)-(dodec-2′-en-1′-yl) succinoyl] chitosan: Synthesis and properties. React. Funct. Polym. 2008, 68, 436–445. [Google Scholar] [CrossRef]
- Philippova, O.; Korchagina, E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Anwer, M.A.; Naguib, H.E. Study on the morphological, dynamic mechanical and thermal properties of PLA carbon nanofibre composites. Compos. Part B Eng. 2016, 91, 631–639. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.; Alothman, O.Y.; Abdullah, L.C. Thermomechanical and dynamic mechanical properties of bamboo/woven kenaf mat reinforced epoxy hybrid composites. Compos. Part B Eng. 2019, 163, 165–174. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.A.; Majumdar, A.; Butola, B.S. Harnessing the ductility of polylactic acid/halloysite nanocomposites by synergistic effects of impact modifier and plasticiser. Compos. Part B Eng. 2020, 188, 107845. [Google Scholar] [CrossRef]
- Hassan, M.M.; Koyama, K. Thermomechanical and viscoelastic properties of green composites of PLA using chitin micro-particles as fillers. J. Polym. Res. 2020, 27, 27. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Trongsatitkul, T.; Lourdin, D.; Capron, I. Chitin Pickering emulsion for oil inclusion in composite films. Carbohydr. Polym. 2020, 242, 116366. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Chaudhary, R. Effect of fiber content on thermal behavior and viscoelastic properties of PALF/Epoxy and COIR/Epoxy composites. Mater. Res. Express 2018, 5, 125305. [Google Scholar] [CrossRef]
- Khemakhem, M.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Effect of core–shell acrylate rubber particles on the thermomechanical and physical properties of biocomposites from polylactic acid and olive solid waste. Polym. Eng. Sci. 2018, 58, 894–902. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological behavior of polymer/carbon nanotube composites: An overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Misra, M.; Mohanty, A.K. Polycarbonate biocomposites reinforced with a hybrid filler system of recycled carbon fiber and biocarbon: Preparation and thermomechanical characterization. J. Appl. Polym. Sci. 2018, 135, 46449. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Cheung, H.-Y.; Lau, K.-T.; Xu, C.-L.; Zhao, D.-D.; Li, H.-L. Silkworm silk/poly (lactic acid) biocomposites: Dynamic mechanical, thermal and biodegradable properties. Polym. Degrad. Stab. 2010, 95, 1978–1987. [Google Scholar] [CrossRef]
- Rajesh, C.; Divia, P.; Dinooplal, S.; Unnikrishnan, G.; Purushothaman, E. Dynamic mechanical analysis of nylon 6 fiber-reinforced acrylonitrile butadiene rubber composites. Polym. Polym. Compos. 2021, 29 (Suppl. 9), S1328–S1339. [Google Scholar] [CrossRef]
- Pothan, L.A.; Thomas, S.; Groeninckx, G. The role of fibre/matrix interactions on the dynamic mechanical properties of chemically modified banana fibre/polyester composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1260–1269. [Google Scholar] [CrossRef]
- Pothan, L.A.; Oommen, Z.; Thomas, S. Dynamic mechanical analysis of banana fiber reinforced polyester composites. Compos. Sci. Technol. 2003, 63, 283–293. [Google Scholar] [CrossRef]
- Joseph, B.; Sam, R.M.; Balakrishnan, P.; Maria, H.J.; Gopi, S.; Volova, T.; Fernandes, S.C.M.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Joseph, P.; Mathew, G.; Joseph, K.; Groeninckx, G.; Thomas, S. Dynamic mechanical properties of short sisal fibre reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2003, 34, 275–290. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Mechanical and thermal properties of coconut shell powder filled polylactic acid biocomposites: Effects of the filler content and silane coupling agent. J. Polym. Res. 2012, 19, 9859. [Google Scholar] [CrossRef]
- Mei, W.; Rothenberger, A.J.; Bostwick, J.E.; Rinehart, J.M.; Hickey, R.J.; Colby, R.H. Zwitterions Raise the Dielectric Constant of Soft Materials. Phys. Rev. Lett. 2021, 127, 228001. [Google Scholar] [CrossRef] [PubMed]
- Šegatin, N.; Pajk Žontar, T.; Poklar Ulrih, N. Dielectric Properties and Dipole Moment of Edible Oils Subjected to ‘Frying’Thermal Treatment. Foods 2020, 9, 900. [Google Scholar] [CrossRef]
- Shah, Z.M.; Khanday, F.A. Analysis of disordered dynamics in polymer nanocomposite dielectrics for the realization of fractional-order capacitor. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 266–273. [Google Scholar] [CrossRef]
- Bhattacharjee, S.K.; Chakraborty, G.; Kashyap, S.P.; Gupta, R.; Katiyar, V. Study of the thermal, mechanical and melt rheological properties of rice straw filled poly (butylene succinate) bio-composites through reactive extrusion process. J. Polym. Environ. 2021, 29, 1477–1488. [Google Scholar] [CrossRef]
- Ojha, S.K.; Purkait, P.; Chatterjee, B.; Chakravorti, S. Application of Cole–Cole model to transformer oil-paper insulation considering distributed dielectric relaxation. High Volt. 2019, 4, 72–79. [Google Scholar] [CrossRef]
- Jamal, M.; Giustozzi, F. Low-content crumb rubber modified bitumen for improving Australian local roads condition. J. Clean. Prod. 2020, 271, 122484. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef]
- Habibi, Y.; Aouadi, S.; Raquez, J.-M.; Dubois, P. Effects of interfacial stereocomplexation in cellulose nanocrystal-filled polylactide nanocomposites. Cellulose 2013, 20, 2877–2885. [Google Scholar] [CrossRef]
- Pirani, S.I.; Krishnamachari, P.; Hashaikeh, R. Optimum loading level of nanoclay in PLA nanocomposites: Impact on the mechanical properties and glass transition temperature. J. Thermoplast. Compos. Mater. 2014, 27, 1461–1478. [Google Scholar] [CrossRef]
- Maubane, L.; Ray, S.S.; Jalama, K. The effect of starch amylose content on the morphology and properties of melt-processed butyl-etherified starch/poly [(butylene succinate)-co-adipate] blends. Carbohydr. Polym. 2017, 155, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, mechanical and thermal properties of poly (lactic acid)(PLA)/cellulose nanofibrils (CNF) composites nanofiber for tissue engineering. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Feng, D.; Zhao, J.; Sun, J.; Li, D. Comparative Study on Properties of Polylactic Acid Nanocomposites with Cellulose and Chitin Nanofibers Extracted from Different Raw Materials. J. Nanomater. 2017, 2017, 7193263. [Google Scholar] [CrossRef]
- Surya, I.; Olaiya, N.; Rizal, S.; Zein, I.; Sri Aprilia, N.; Hasan, M.; Yahya, E.B.; Sadasivuni, K.; Abdul Khalil, H. Plasticizer Enhancement on the Miscibility and Thermomechanical Properties of Polylactic Acid-Chitin-Starch Composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Mihai, M.; Huneault, M.A.; Favis, B.D.; Li, H. Extrusion foaming of semi-crystalline PLA and PLA/thermoplastic starch blends. Macromol. Biosci. 2007, 7, 907–920. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Abeyratne, C.; Bangamuwa, O.M.; Ludowyke, N.; Dahanayake, D.; Gunasekara, S.; de Silva, N.; de Silva, R.M.; de Silva, K.N. In-situ formation of supramolecular aggregates between chitin nanofibers and silver nanoparticles. Carbohydr. Polym. 2017, 173, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Irvin, C.W.; Satam, C.C.; Meredith, J.C.; Shofner, M.L. Mechanical reinforcement and thermal properties of PVA tricomponent nanocomposites with chitin nanofibers and cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2019, 116, 147–157. [Google Scholar] [CrossRef]
- Awale, R.J.; Ali, F.B.; Azmi, A.S.; Puad, N.I.M.; Anuar, H.; Hassan, A. Enhanced flexibility of biodegradable polylactic acid/starch blends using epoxidized palm oil as plasticizer. Polymers 2018, 10, 977. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Pal, A.K.; Woo, E.M.; Katiyar, V. Effects of Amphiphilic Chitosan on Stereocomplexation and Properties of Poly (lactic acid) Nano-biocomposite. Sci. Rep. 2018, 8, 4351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin nanofibrils in poly (lactic acid)(PLA) nanocomposites: Dispersion and thermo-mechanical properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G. Properties and skin compatibility of films based on poly (lactic acid)(PLA) bionanocomposites incorporating chitin nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Wang, Z.; Guo, Z.; Ma, Y.; Wang, C.; Tan, H.; Zhang, Y. Study on the properties of PLA/PBAT composite modified by nanohydroxyapatite. J. Mater. Res. Technol. 2020, 9, 11895–11904. [Google Scholar] [CrossRef]
- Phua, Y.; Lau, N.; Sudesh, K.; Chow, W.; Ishak, Z.M. Biodegradability studies of poly (butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: Effects of clay loading and compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Qu, A.-T.; Ma, R.-J.; Li, A.; Zhang, Z.-K.; Shang, Z.-Q.; Zhang, Y.-F.; Bu, L.-X.; An, Y.-L. PH-responsive micelles from a blend of PEG-b-PLA and PLA-b-PDPA block copolymers: Core protection against enzymatic degradation. Chin. J. Polym. Sci. 2018, 36, 1262–1268. [Google Scholar] [CrossRef]
- Sreekumar, P.; Thomas, S.P.; marc Saiter, J.; Joseph, K.; Unnikrishnan, G.; Thomas, S. Effect of fiber surface modification on the mechanical and water absorption characteristics of sisal/polyester composites fabricated by resin transfer molding. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1777–1784. [Google Scholar] [CrossRef]













| S/N | PLA (wt%) | Amphiphilic Chitin | Starch (wt%) |
|---|---|---|---|
| Neat PLA | 100 | 0 | 0 |
| PS8 | 92 | 0 | 8 |
| PC2S6 | 92 | 2 | 6 |
| PC4S4 | 92 | 4 | 4 |
| PC6S2 | 92 | 6 | 2 |
| PC8 | 92 | 8 | 0 |
| Sample | Coefficient of Composite (Ccomp) | Coefficient of Resin (Cresin) | Coefficient of Effectiveness (C) |
|---|---|---|---|
| P8S | 8.67 | 14.00 | 0.62 |
| P6S2C | 12.00 | 14.00 | 0.86 |
| P4S4C | 3.93 | 14.00 | 0.28 |
| P2S6C | 10.87 | 14.00 | 0.78 |
| P8C | 12.87 | 14.00 | 0.92 |
| Sample | Glass Transition Temperature (°C) | |||
|---|---|---|---|---|
| 0.1 | 1 HZ | 5 HZ | 10 HZ | |
| PLA | 72.8 | 73.1 | 73.1 | 77.6 |
| P8S | 72.1 | 72.1 | 74.4 | 76.8 |
| P6S2C | 71.8 | 71.8 | 74.5 | 76.4 |
| P4S4C | 83.0 | 83.9 | 86.3 | 88.1 |
| P2S6C | 72.4 | 72.3 | 74.6 | 77.1 |
| P8C | 76.7 | 76.7 | 74.4 | 72.3 |
| Sample | Tg (C) | Tc (C) | Tm (C) | |
|---|---|---|---|---|
| PLA | 62.5 | 107.5 | 162.3 | - |
| P8S | 60.6 | 106.7 | 164.3 | - |
| P6S2C | 59.8 | 106.5 | 163.0 | 177.3 |
| P4S4C | 55.6 | - | 152.3 | 155.3 |
| P2S6C | 53.3 | - | 150.2 | - |
| P8C | 61.6 | 106.2 | 163.1 | - |
| Samples | To (°C) | Tp (°C) |
|---|---|---|
| PLA | 299.8 | 375.2 |
| P8S | 302.1 | 371.3 |
| P6S2C | 286.1 | 368.8 |
| P4S4C | 287.4 | 349.1 |
| P2S6C | 283.6 | 346.2 |
| P8C | 281.3 | 339.3 |
| Composite | Plasticiser | Tensile Strength MPa | Tensile Modulus MPa | Method | Guage Length (mm) | Reference |
|---|---|---|---|---|---|---|
| PLA/starch | none | 45.5 | 1078 | Hot press | 25 | [13] |
| PLA/starch | Epoxidized Palm Oil | 62.5 | 2750 | Hot press | 50 | [65] |
| Plasticised PLA/starch | Gelatin | 44.7 | 1175 | Hot press | 25 | [13] |
| PLA/amphiphilic chitin | none | 63 | 2800 | Melt extrusion | 50 | [66] |
| PLA/nano chitin | poly(ethylene glycol) | 58 | 3500 | Melt extrusion | 50 | [67] |
| PLA/amphiphilic chitin/starch | Chloroform | 87 | 7600 | Melt extrusion and Compression moulding | 50 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaiya, N.G.; Maraveas, C.; Salem, M.A.; Raja, S.; Rashedi, A.; Alzahrani, A.Y.; El-Bahy, Z.M.; Olaiya, F.G. Viscoelastic and Properties of Amphiphilic Chitin in Plasticised Polylactic Acid/Starch Biocomposite. Polymers 2022, 14, 2268. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112268
Olaiya NG, Maraveas C, Salem MA, Raja S, Rashedi A, Alzahrani AY, El-Bahy ZM, Olaiya FG. Viscoelastic and Properties of Amphiphilic Chitin in Plasticised Polylactic Acid/Starch Biocomposite. Polymers. 2022; 14(11):2268. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112268
Chicago/Turabian StyleOlaiya, N. G., C. Maraveas, Mohamed A. Salem, S. Raja, Ahmad Rashedi, Abdullah Y. Alzahrani, Zeinhom M. El-Bahy, and Funmilayo G. Olaiya. 2022. "Viscoelastic and Properties of Amphiphilic Chitin in Plasticised Polylactic Acid/Starch Biocomposite" Polymers 14, no. 11: 2268. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14112268