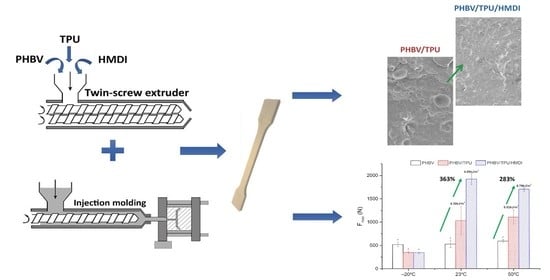
In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Morphology Characterization
2.4. Thermal Characterization
2.5. Rheology
2.6. Mechanical Characterization
2.7. Biodegradation
3. Results
3.1. Morphology Characterization
- Increase in toughening, as the fraction of particles between 0.2 and 0.4 µm increases and the number of big particles (d > 0.6 µm) decreases.
- Increase in melt viscosity due to the increased interfacial adhesion between PHBV and TPU.
3.2. Thermal Characterization
3.2.1. Thermogravimetric Analysis
3.2.2. Differential Scanning Calorimetry
3.2.3. Dynamic Mechanical Analysis
3.3. Rheology
3.3.1. Plate-Plate Rheology
3.3.2. Capillary Rheometry
3.4. Mechanical Characterization
3.4.1. Tensile Test
3.4.2. Flexural Tests
3.4.3. Tensile Creep
3.4.4. Dart Drop Impact Tests
3.5. Biodegradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe: The Facts 2020-An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Frankfurt, Germany, 2020.
- A European Strategy for Plastics in a Circular Economy. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Union: Brussels, Belgium, 2018. [Google Scholar]
- European Bioplastics. Global production capacities of bioplastics 2019–2025 Global Production Capacities of Bioplastics 2020 (by Material type) Global Production Capacities of Bioplastics 2025 (by Material Type); European Bioplastics: Hamburg, Germany, 2020. [Google Scholar]
- Balart, R.; Montanes, N.; Dominici, F.; Boronat, T.; Torres-Giner, S. Environmentally Friendly Polymers and Polymer Composites. Materials 2020, 13, 4892. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; de Nisi, P.; Tambone, F.; Adani, F. The role of waste management in reducing bioplastics’ leakage into the environment: A review. Bioresour. Technol. 2021, 337, 125459. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Figueroa-Lopez, K.J.; Melendez-Rodriguez, B.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Emerging Trends in Biopolymers for Food Packaging. In Sustainable Food Packaging Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 1–33. [Google Scholar]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Silva, J.B.; Pereira, J.R.; Marreiros, B.C.; Reis, M.A.M.; Freitas, F. Microbial production of medium-chain length polyhydroxyalkanoates. Process Biochem. 2021, 102, 393–407. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Koller, M. Poly(hydroxyalkanoates) for food packaging—Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Hussein, M.A. Properties and Applications of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biocomposites. J. Polym. Environ. 2021, 29, 1010–1030. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Angellier-Coussy, H.; Guillard, V.; Gontard, N. Vegetal fiber-based biocomposites: Which stakes for food packaging applications? J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Rosa, M.d.F.; Cioffi, M.O.H.; Benini, K.C.C.d.C.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal fibers in polymeric composites: A review. Polímeros 2015, 25, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Zytner, P.; Wu, F.; Misra, M.; Mohanty, A.K. Toughening of Biodegradable Poly(3-hydroxybutyrate- co-3-hydroxyvalerate)/Poly(Є-caprolactone) Blends by in Situ Reactive Compatibilization. ACS Omega 2020, 5, 14900–14910. [Google Scholar] [CrossRef]
- Wu, B.; Zeng, Q.; Niu, D.; Yang, W.; Dong, W.; Chen, M.; Ma, P. Design of Supertoughened and Heat-Resistant PLLA/Elastomer Blends by Controlling the Distribution of Stereocomplex Crystallites and the Morphology. Macromolecules 2019, 52, 1092–1103. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Tiwary, P.; Najafi, N.; Kontopoulou, M. Advances in peroxide-initiated graft modification of thermoplastic biopolyesters by reactive extrusion. Can. J. Chem. Eng. 2021, 99, 1870–1884. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 608–615. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.C.C.; Carreau, P.J.J.; Wood-Adams, P.M. Control of thermal degradation of polylactide (PLA)-clay nanocomposites using chain extenders. Polym. Degrad. Stab. 2012, 97, 554–565. [Google Scholar] [CrossRef]
- Wu, S. Phase structure and adhesion in polymer blends: A criterion for rubber toughening. Polymer 1985, 26, 1855–1863. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Paul, D.R. Notched impact behavior of polymer blends: Part 1: New model for particle size dependence. Polymer 2009, 50, 5539–5548. [Google Scholar] [CrossRef] [Green Version]
- Bucknall, C.B.; Paul, D.R. Notched impact behaviour of polymer blends: Part 2: Dependence of critical particle size on rubber particle volume fraction. Polymer 2013, 54, 320–329. [Google Scholar] [CrossRef]
- Dompas, D.; Groeninckx, G. Toughening behaviour of rubber-modified thermoplastic polymers involving very small rubber particles: 1. A criterion for internal rubber cavitation. Polymer 1994, 35, 4743–4749. [Google Scholar] [CrossRef]
- Muratoglu, O.K.; Argon, A.S.; Cohen, R.E.; Weinberg, M. Toughening mechanism of rubber-modified polyamides. Polymer 1995, 36, 921–930. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Jiang, L.; Qiao, J. Advances in toughened polymer materials by structured rubber particles. Prog. Polym. Sci. 2019, 98, 101160. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; González-Ausejo, J.; Lagarón, J.M.; Cabedo, L. Biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/thermoplastic polyurethane blends with improved mechanical and barrier performance. Polym. Degrad. Stab. 2016, 132, 52–61. [Google Scholar] [CrossRef]
- Yu, R.L.; Zhang, L.S.; Feng, Y.H.; Zhang, R.Y.; Zhu, J. Improvement in toughness of polylactide by melt blending with bio-based poly(ester)urethane. Chinese J. Polym. Sci. 2014, 32, 1099–1110. [Google Scholar] [CrossRef]
- Zhang, H.C.; Kang, B.H.; Chen, L.S.; Lu, X. Enhancing toughness of poly (lactic acid)/Thermoplastic polyurethane blends via increasing interface compatibility by polyurethane elastomer prepolymer and its toughening mechanism. Polym. Test. 2020, 87, 106521. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PBS and PHB/PBSA blends: Morphology, phase composition and modelling of elastic modulus. Polym. Int. 2021, 71, 47–56. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In Situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Misra, M.; Mohanty, A.K. Toughened Sustainable Green Composites from Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Based Ternary Blends and Miscanthus Biofiber. ACS Sustain. Chem. Eng. 2014, 2, 2345–2354. [Google Scholar] [CrossRef]
- Matta, A.K.; Rao, R.U.; Suman, K.N.S.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Safont, E.L.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. PHBV/TPU/cellulose compounds for compostable injection molded parts with improved thermal and mechanical performance. J. Appl. Polym. Sci. 2019, 136, 47257. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Cabedo, L.; Gamez-Perez, J. Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications. Int. J. Mol. Sci. 2018, 19, 2102. [Google Scholar] [CrossRef] [Green Version]
- Auras, R.A.; Singh, S.P.; Singh, J.J. Evaluation of oriented poly(lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag. Technol. Sci. 2005, 18, 207–216. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Patil, A.; Patel, A.; Purohit, R. An overview of Polymeric Materials for Automotive Applications. Mater. Today Proc. 2017, 4, 3807–3815. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Power law (Ostwald-deWaele model). Encycl. Dict. Polym. 2007, 2007, 781. [CrossRef]
- González-Ausejo, J.; Sánchez-Safont, E.; Lagarón, J.M.; Balart, R.; Cabedo, L.; Gámez-Pérez, J. Compatibilization of poly(3-hydroxybutyrate- co -3-hydroxyvalerate)-poly(lactic acid) blends with diisocyanates. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable polymer blends and composites: An overview. Polym. Sci.-Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- Takayama, T.; Todo, M. Improvement of impact fracture properties of PLA/PCL polymer blend due to LTI addition. J. Mater. Sci. 2006, 41, 4989–4992. [Google Scholar] [CrossRef]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.M.; Grohens, Y.; Lopez-Cuesta, J.M. Synergistic effect of Compatibilizer and cloisite 30B on the functional properties of poly(3-hydroxybutyrateco- 3-hydroxyvalerate)/Polylactide blends. Polym. Eng. Sci. 2014, 54, 2239–2251. [Google Scholar] [CrossRef]
- Abate, R.; Ballistreri, A.; Montando, G.; Impallomeni, G. Thermal Degradation of Microbial Poly(4-hydroxybutyrate). Macromolecules 1994, 27, 332–336. [Google Scholar] [CrossRef]
- Menczel, J.D. Dynamic mechanical analysis (DMA) in fiber research. In Thermal Analysis of Textiles and Fibers; Woodhead Publishing: Sawston, UK, 2020; pp. 95–104. [Google Scholar]
- Essabir, H.; El Mechtali, F.Z.; Nekhlaoui, S.; Raji, M.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Compatibilization of PA6/ABS blend by SEBS-g-MA: Morphological, mechanical, thermal, and rheological properties. Int. J. Adv. Manuf. Technol. 2020, 110, 1095–1111. [Google Scholar] [CrossRef]
- D’Anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef] [Green Version]
- Karami, S.; Nazockdast, H.; Ahmadi, Z.; Rabolt, J.F.; Noda, I.; Chase, D.B. Microstructure effects on the rheology of nanoclay-filled PHB/LDPE blends. Polym. Compos. 2019, 40, 4125–4134. [Google Scholar] [CrossRef]
- Hoseini, M.; Haghtalab, A.; Famili, M.H.N. Rheology and morphology study of immiscible linear low-density polyethylene/poly(lactic acid) blends filled with nanosilica particles. J. Appl. Polym. Sci. 2017, 134, 45526. [Google Scholar] [CrossRef]
- Cunha, M.; Fernandes, B.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Film blowing of PHBV blends and PHBV-based multilayers for the production of biodegradable packages. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef] [Green Version]
- Kopinke, F.D.; Mackenzie, K. Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(β-hydroxybutyric acid). J. Anal. Appl. Pyrolysis 1997, 40–41, 43–53. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zhu, M.-F.; Wu, W.-H.; Qin, Z.-Y. Reducing the formation of six-membered ring ester during thermal degradation of biodegradable PHBV to enhance its thermal stability. Polym. Degrad. Stab. 2009, 94, 18–24. [Google Scholar] [CrossRef]
- Kasgoz, A. Mechanical, Tensile Creep and Viscoelastic Properties of Thermoplastic Polyurethane/Polycarbonate Blends. Fibers Polym. 2021, 22, 295–305. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Niaounakis, M. Thermomechanical properties and rheological behavior of biodegradable composites. Polym. Compos. 2014, 35, 1140–1149. [Google Scholar] [CrossRef]
- Fekete, I.; Ronkay, F.; Lendvai, L. Highly toughened blends of poly(lactic acid) (PLA) and natural rubber (NR) for FDM-based 3D printing applications: The effect of composition and infill pattern. Polym. Test. 2021, 99, 107205. [Google Scholar] [CrossRef]
- Panda, B.P.; Mohanty, S.; Nayak, S.K. Mechanism of Toughening in Rubber Toughened Polyolefin—A Review. Polym.-Plast. Technol. Eng. 2015, 54, 462–473. [Google Scholar] [CrossRef]
- Suthapakti, K.; Molloy, R.; Punyodom, W.; Nalampang, K.; Leejarkpai, T.; Topham, P.D.; Tighe, B.J. Biodegradable Compatibilized Poly(l-lactide)/Thermoplastic Polyurethane Blends: Design, Preparation and Property Testing. J. Polym. Environ. 2018, 26, 1818–1830. [Google Scholar] [CrossRef]
- Alhanish, A.; Abu Ghalia, M. Biobased Thermoplastic Polyurethanes and Their Capability to Biodegradation. In Eco-Friendly Adhesives for Wood and Natural Fiber Composites, Composites Science and Technology; Springer: Singapore, 2021; pp. 85–104. [Google Scholar]












| Sample | PHBV (wt%) | TPU (phr) | HMDI (phr) |
|---|---|---|---|
| PHBV | 100 | 0 | 0 |
| PHBV/TPU | 100 | 30 | 0 |
| PHBV/TPU/HMDI | 100 | 30 | 1 |
| Samples | Td (°C) | T5% (°C) |
|---|---|---|
| PHBV | 298.2 | 278.7 |
| PHBV/TPU | 297.0 | 279.5 |
| PHVB/TPU/HMDI | 296.3 | 279.2 |
| Sample | Tm (°C) | ΔHm (J/g) | χc (%) | Tc (°C) | ΔHc |
|---|---|---|---|---|---|
| PHBV | 173.1 | 97.4 | 66.7 | 124.0 | 91.0 |
| PHBV/TPU | 171.4 | 72.2 | 64.3 | 113.0 | 65.6 |
| PHVB/TPU/HMDI | 171.5 | 70.7 | 63.4 | 112.3 | 68.7 |
| Samples | Injection Pressure (bar) | Consistency Coefficient, K, (Pa·sn) | Flow-Behavior Index, n (−) |
|---|---|---|---|
| PHBV | 450 | 18,160 | 0.296 |
| PHBV/TPU | 520 | 29,030 | 0.266 |
| PHVB/TPU/HMDI | 590 | 42,950 | 0.243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego-Aguilar, K.; Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts. Polymers 2022, 14, 2337. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122337
Samaniego-Aguilar K, Sánchez-Safont E, Arrillaga A, Anakabe J, Gamez-Perez J, Cabedo L. In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts. Polymers. 2022; 14(12):2337. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122337
Chicago/Turabian StyleSamaniego-Aguilar, Kerly, Estefanía Sánchez-Safont, Alex Arrillaga, Jon Anakabe, Jose Gamez-Perez, and Luis Cabedo. 2022. "In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts" Polymers 14, no. 12: 2337. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122337