Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Polymeric Scaffolds
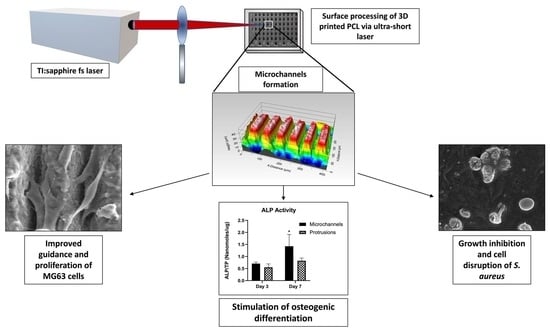
2.2. Surface Modification by Femtosecond-Laser Machining
2.3. Characterization of fs-Laser-Processed Scaffolds
2.3.1. Assessment of fs Laser Derived Topography Features and Their Effects on the Morphology and Cell Growth Pattern of S. aureus and MG-63 Osteoblastic Cell Lines via Microscopic Techniques
2.3.2. Analysis of Wettability and Surface Energy Changes in Relation to fs-Laser Processing
2.4. Degradation Test in Phosphate Buffer Saline
2.5. In Vitro Cytocompatibility Assessment
2.5.1. Resazurin Assay
2.5.2. Alkaline Phosphatase (ALP) Activity
2.6. Antimicrobial Activity
2.7. Statistical Analysis
3. Results
3.1. Inducing Morphological Changes in the Surface Profile of the 3D-Printed PCL Scaffolds by fs-Laser Micromachining
3.2. Hydrophobic Behaviour of fs-Laser-Treated PCL Scaffolds
3.3. Change in pH of PBS in Accordance with PCL Degradation
3.4. Cytocompatibility
3.5. Antibacterial Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matassi, F.; Botti, A.; Sirleo, L.; Carulli, C.; Innocenti, M. Porous Metal for Orthopedics Implants. Clin. Cases Miner. Bone Metab. 2013, 10, 111–115. [Google Scholar] [PubMed]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable Polymer Nanocomposites for Ligament/Tendon Tissue Engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Jahani, H.; Jalilian, F.A.; Wu, C.; Kaviani, S.; Soleimani, M.; Abbasi, N.; Ou, K.; Hosseinkhani, H. Controlled Surface Morphology and Hydrophilicity of Polycaprolactone toward Selective Differentiation of Mesenchymal Stem Cells to Neural like Cells. J. Biomed. Mater. Res. A 2015, 103, 1875–1881. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wootton, D.M.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.I.; Zhou, J. Mechanical Study of Polycaprolactone-Hydroxyapatite Porous Scaffolds Created by Porogen-Based Solid Freeform Fabrication Method. J. Appl. Biomater. Funct. Mater. 2014, 12, 145–154. [Google Scholar] [CrossRef]
- Mondrinos, M.; Dembzynski, R.; Lu, L.; Byrapogu, V.; Wootton, D.; Lelkes, P.; Zhou, J. Porogen-Based Solid Freeform Fabrication of Polycaprolactone–Calcium Phosphate Scaffolds for Tissue Engineering. Biomaterials 2006, 27, 4399–4408. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Nag, T.C. A Comparison of Bacterial Adhesion and Biofilm Formation on Commonly Used Orthopaedic Metal Implant Materials: An In Vitro Study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Reis, A.C.D. Bacterial Adhesion to Biomaterials: What Regulates This Attachment? A Review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The Interaction of Cells and Bacteria with Surfaces Structured at the Nanometre Scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct Femtosecond Laser Surface Nano/Microstructuring and Its Applications: Direct Femtosecond Laser Surface Nano/Microstructuring and Its Applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Krüger, J.; Kautek, W. Ultrashort Pulse Laser Interaction with Dielectrics and Polymers. In Polymers and Light; Lippert, T.K., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 168, pp. 247–290. ISBN 978-3-540-40471-2. [Google Scholar]
- Toyserkani, E.; Rasti, N. Ultrashort Pulsed Laser Surface Texturing. In Laser Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 441–453. ISBN 978-1-78242-074-3. [Google Scholar]
- Siddiquie, R.Y.; Gaddam, A.; Agrawal, A.; Dimov, S.S.; Joshi, S.S. Anti-Biofouling Properties of Femtosecond Laser-Induced Submicron Topographies on Elastomeric Surfaces. Langmuir 2020, 36, 5349–5358. [Google Scholar] [CrossRef]
- Chen, C.; Enrico, A.; Pettersson, T.; Ek, M.; Herland, A.; Niklaus, F.; Stemme, G.; Wågberg, L. Bactericidal Surfaces Prepared by Femtosecond Laser Patterning and Layer-by-Layer Polyelectrolyte Coating. J. Colloid Interface Sci. 2020, 575, 286–297. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating Superhydrophobic and Antibacterial Surfaces on Gold by Femtosecond Laser Pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef]
- Martínez-Calderon, M.; Rodríguez, A.; Dias-Ponte, A.; Morant-Miñana, M.C.; Gómez-Aranzadi, M.; Olaizola, S.M. Femtosecond Laser Fabrication of Highly Hydrophobic Stainless Steel Surface with Hierarchical Structures Fabricated by Combining Ordered Microstructures and LIPSS. Appl. Surf. Sci. 2016, 374, 81–89. [Google Scholar] [CrossRef]
- McHale, G.; Newton, M.I.; Shirtcliffe, N.J. Dynamic Wetting and Spreading and the Role of Topography. J. Phys. Condens. Matter 2009, 21, 464122. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Ostrowska, B.; Zhelyazkova, A.; Święszkowski, W.; Trifonov, A.; Declercq, H.; Nathala, C.; Szlazak, K.; Lojkowski, M.; Husinsky, W.; et al. Improving Osteoblasts Cells Proliferation via Femtosecond Laser Surface Modification of 3D-Printed Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering Applications. Appl. Phys. A 2018, 124, 413. [Google Scholar] [CrossRef]
- Rožić, M.; Šegota, N.; Vukoje, M.; Kulčar, R.; Šegota, S. Description of Thermochromic Offset Prints Morphologies Depending on Printing Substrate. Appl. Sci. 2020, 10, 8095. [Google Scholar] [CrossRef]
- Daskalova, A.; Filipov, E.; Angelova, L.; Stefanov, R.; Tatchev, D.; Avdeev, G.; Sotelo, L.; Christiansen, S.; Sarau, G.; Leuchs, G.; et al. Ultra-Short Laser Surface Properties Optimization of Biocompatibility Characteristics of 3D Poly-ε-Caprolactone and Hydroxyapatite Composite Scaffolds. Materials 2021, 14, 7513. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-C.; Song, S.-M.; Tsai, P.-H.; Nien, Y.-Y.; Jang, J.S.-C.; Cheng, C.-K.; Chen, C.-H. Relationship between the Surface Roughness of Biodegradable Mg-Based Bulk Metallic Glass and the Osteogenetic Ability of MG63 Osteoblast-Like Cells. Materials 2020, 13, 1188. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in Superhydrophobic Surface Development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Lins, L.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic Liquid as Surfactant Agent of Hydrotalcite: Influence on the Final Properties of Polycaprolactone Matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.; Choi, S.-O.; Kim, J. Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef]
- Arakawa, C.K.; DeForest, C.A. Polymer Design and Development. In Biology and Engineering of Stem Cell Niches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 295–314. ISBN 978-0-12-802734-9. [Google Scholar]
- Rangel, A.; Nguyen, T.N.; Egles, C.; Migonney, V. Different Real-time Degradation Scenarios of Functionalized Poly(Ε-caprolactone) for Biomedical Applications. J. Appl. Polym. Sci. 2021, 138, 50479. [Google Scholar] [CrossRef]
- Taylor, R.L.; Verran, J.; Lees, G.C.; Ward, A.J.P. The Influence of Substratum Topography on Bacterial Adhesion to Polymethyl Methacrylate. J. Mater. Sci. Mater. Med. 1998, 9, 17–22. [Google Scholar] [CrossRef]
- Wang, Y.; Subbiahdoss, G.; Swartjes, J.; van der Mei, H.C.; Busscher, H.J.; Libera, M. Length-Scale Mediated Differential Adhesion of Mammalian Cells and Microbes. Adv. Funct. Mater. 2011, 21, 3916–3923. [Google Scholar] [CrossRef]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef]
- Toosi, S.F.; Moradi, S.; Hatzikiriakos, S.G. Fabrication of Micro/Nano Patterns on Polymeric Substrates Using Laser Ablation Methods to Control Wettability Behaviour: A Critical Review. Rev. Adhes. Adhes. 2017, 5, 55–78. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Antunes, J.C.; Martins, M.C.L.; Barbosa, M.A. Fundamentals of Protein and Cell Interactions in Biomaterials. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–27. ISBN 978-0-08-100803-4. [Google Scholar]
- Yeong, W.Y.; Yu, H.; Lim, K.P.; Ng, K.L.G.; Boey, Y.C.F.; Subbu, V.S.; Tan, L.P. Multiscale Topological Guidance for Cell Alignment via Direct Laser Writing on Biodegradable Polymer. Tissue Eng. Part C Methods 2010, 16, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wong, Y.S.; Wen, F.; Ng, K.W.; Ng, G.K.L.; Venkatraman, S.S.; Boey, F.Y.C.; Tan, L.P. Human Mesenchymal Stem-Cell Behaviour On Direct Laser Micropatterned Electrospun Scaffolds with Hierarchical Structures. Macromol. Biosci. 2013, 13, 299–310. [Google Scholar] [CrossRef]
- Ortiz, R.; Moreno-Flores, S.; Quintana, I.; Vivanco, M.; Sarasua, J.R.; Toca-Herrera, J.L. Ultra-Fast Laser Microprocessing of Medical Polymers for Cell Engineering Applications. Mater. Sci. Eng. C 2014, 37, 241–250. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.; Ru, J.; Wang, F.; Xu, M.; Zhou, Q.; Stanikzai, H.; Yerlan, I.; Xu, Z.; Niu, Y.; et al. Creating Micro-Submicro Structure and Grafting Hydroxyl Group on PEEK by Femtosecond Laser and Hydroxylation to Synergistically Activate Cellular Response. Mater. Des. 2021, 199, 109413. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, Behavior, and Differentiation of Osteoblasts on Surfaces of Different Microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic Differentiation of Human Mesenchymal Stem Cells in the Absence of Osteogenic Supplements: A Surface-Roughness Gradient Study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef]
- Lee, B.L.-P.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulos, C.; Li, S. Femtosecond Laser Ablation Enhances Cell Infiltration into Three-Dimensional Electrospun Scaffolds. Acta Biomater. 2012, 8, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, F.; Wong, Y.S.; Boey, F.Y.C.; Subbu, V.S.; Leong, D.T.; Ng, K.W.; Ng, G.K.L.; Tan, L.P. Direct Laser Machining-Induced Topographic Pattern Promotes up-Regulation of Myogenic Markers in Human Mesenchymal Stem Cells. Acta Biomater. 2012, 8, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Nathala, C.S.R.; Bliznakova, I.; Stoyanova, E.; Zhelyazkova, A.; Ganz, T.; Lueftenegger, S.; Husinsky, W. Controlling the Porosity of Collagen, Gelatin and Elastin Biomaterials by Ultrashort Laser Pulses. Appl. Surf. Sci. 2014, 292, 367–377. [Google Scholar] [CrossRef]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Wu, Y.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and Characterization of Electrospun Poly(ε-Caprolactone)/Gelatin Nanofiber Tissue Scaffolds by Femtosecond Laser Ablation for Tissue Engineering Applications. Biotechnol. Bioeng. 2011, 108, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lim, Y.C.; Farson, D.F.; Powell, H.M.; Lannutti, J.J. Vascular Wall Engineering Via Femtosecond Laser Ablation: Scaffolds with Self-Containing Smooth Muscle Cell Populations. Ann. Biomed. Eng. 2011, 39, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of Surface Topography on Bacterial Adhesion: A Review. Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef]
- Wu, X.; Ao, H.; He, Z.; Wang, Q.; Peng, Z. Surface Modification of Titanium by Femtosecond Laser in Reducing Bacterial Colonization. Coatings 2022, 12, 414. [Google Scholar] [CrossRef]
- Shaikh, S.; Kedia, S.; Singh, D.; Subramanian, M.; Sinha, S. Surface Texturing of Ti6Al4V Alloy Using Femtosecond Laser for Superior Antibacterial Performance. J. Laser Appl. 2019, 31, 022011. [Google Scholar] [CrossRef]
- Schwibbert, K.; Menzel, F.; Epperlein, N.; Bonse, J.; Krüger, J. Bacterial Adhesion on Femtosecond Laser-Modified Polyethylene. Materials 2019, 12, 3107. [Google Scholar] [CrossRef]
- Valle, J.; Burgui, S.; Langheinrich, D.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Lasagni, A.; Lasa, I. Evaluation of Surface Microtopography Engineered by Direct Laser Interference for Bacterial Anti-Biofouling. Macromol. Biosci. 2015, 15, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, Y.; Ge, X.; Leng, Y. Control of Bacterial Adhesion and Growth on Honeycomb-like Patterned Surfaces. Colloids Surf. B Biointerfaces 2015, 135, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Leng, Y.; Lu, X.; Ren, F.; Wang, K.; Ding, Y.; Yang, M. Bacterial Responses to Periodic Micropillar Array: Bacterial Responses to Periodic Micropillar Array. J. Biomed. Mater. Res. A 2015, 103, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Pacha-Olivenza, M.Á.; Tejero, R.; Fernández-Calderón, M.C.; Anitua, E.; Troya, M.; González-Martín, M.L. Relevance of Topographic Parameters on the Adhesion and Proliferation of Human Gingival Fibroblasts and Oral Bacterial Strains. BioMed Res. Int. 2019, 2019, 8456342. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipov, E.; Angelova, L.; Vig, S.; Fernandes, M.H.; Moreau, G.; Lasgorceix, M.; Buchvarov, I.; Daskalova, A. Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism. Polymers 2022, 14, 2382. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122382
Filipov E, Angelova L, Vig S, Fernandes MH, Moreau G, Lasgorceix M, Buchvarov I, Daskalova A. Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism. Polymers. 2022; 14(12):2382. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122382
Chicago/Turabian StyleFilipov, Emil, Liliya Angelova, Sanjana Vig, Maria Helena Fernandes, Gerard Moreau, Marie Lasgorceix, Ivan Buchvarov, and Albena Daskalova. 2022. "Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism" Polymers 14, no. 12: 2382. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122382