Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ribonuclease
2.3. Preparation of Initial Solutions
2.4. Preparation of Polysaccharide Microspheres
2.5. Enzyme Release from Hydrogels and RNase Content in Microspheres
2.6. Cytotoxicity Assay
2.7. Scanning Electron Microscopy
2.8. Fluorescence Measurements
2.9. Water Swelling Capacity of Microspheres
3. Results
3.1. Structural Features of Alginate Microspheres and Water Swelling
3.2. Influence of Alginate on Enzyme Structure
3.3. Alginate Microsphere Stability and Enzyme Release
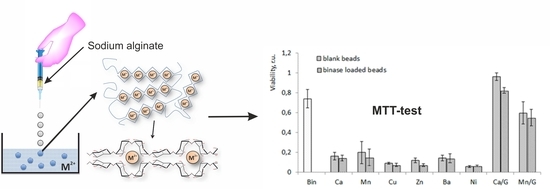
3.4. Cytotoxicity of Alginate Microspheres towards Tumor Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahia, L.H.; Chirani, N.; Motta, F.L.; Chirani, S.; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Bogdanova, L.R.; Makarova, A.O.; Zueva, O.S.; Zakharova, L.Y.; Zuev, Y.F. Encapsulation of Diagnostic Dyes in the Polysaccharide Matrix Modified by Carbon Nanotubes. Russ. Chem. Bull. 2020, 69, 590–595. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Safarova, E.R.; Zuev, Y.F. Structural Insights in Interactions between RNase from Bacillus Intermedius and Rhamnogalacturonan I from Potato. Carbohydr. Polym. 2021, 251, 117038. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef]
- Singh Chandel, A.K.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of Antiperitoneal Adhesion, Hemostasis, and Operability of Compressed Bilayer Ultrapure Alginate Sponges. Biomater. Adv. 2022, 137, 212825. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular Structure and Properties of κ-Carrageenan-Gelatin Gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Rees, D.A. Structure, Conformation, and Mechanism in the Formation of Polysaccharide Gels and Networks. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Cambridge, MA, USA, 1969; Volume 24, pp. 267–332. ISBN 978-0-12-007224-8. [Google Scholar]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef]
- Mazur, K.; Buchner, R.; Bonn, M.; Hunger, J. Hydration of Sodium Alginate in Aqueous Solution. Macromolecules 2014, 47, 771–776. [Google Scholar] [CrossRef]
- Kouser, R.; Vashist, A.; Zafaryab, M.; Rizvi, M.A.; Ahmad, S. PH-Responsive Biocompatible Nanocomposite Hydrogels for Therapeutic Drug Delivery. ACS Appl. Bio Mater. 2018, 1, 1810–1822. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.; Wu, C. Injectable and Body Temperature Sensitive Hydrogels Based on Chitosan and Hyaluronic Acid for PH Sensitive Drug Release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Huang, H. Review on Magnetic Natural Polymer Constructed Hydrogels as Vehicles for Drug Delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjugate Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Su, Z.; Zhang, K.; Ren, J.; Sun, R.; Wang, X. All-Biomass Fluorescent Hydrogels Based on Biomass Carbon Dots and Alginate/Nanocellulose for Biosensing. ACS Appl. Bio Mater. 2018, 1, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A Magnetic Chitosan Hydrogel for Sustained and Prolonged Delivery of Bacillus Calmette–Guérin in the Treatment of Bladder Cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural Hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- Libonati, M.; Gotte, G.; Vottariello, F. A Novel Biological Actions Acquired by Ribonuclease through Oligomerization. Curr. Pharm. Biotechnol. 2008, 9, 200–209. [Google Scholar] [CrossRef]
- Ardelt, B.; Ardelt, W.; Darzynkiewicz, Z. Cytotoxic Ribonucleases and RNA Interference (RNAi). Cell Cycle 2003, 2, 22–24. [Google Scholar] [CrossRef] [Green Version]
- Ardelt, W.; Ardelt, B.; Darzynkiewicz, Z. Ribonucleases as Potential Modalities in Anticancer Therapy. Eur. J. Pharmacol. 2009, 625, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, E.F.; Ng, T.B. Ribonucleases of Different Origins with a Wide Spectrum of Medicinal Applications. Biochim. Biophys. Acta Rev. Cancer 2011, 1815, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Ribo, M.; Benito, A.; Vilanova, M. Mini-Review: Nucleus-Targeted Ribonucleases as Antitumor Drugs. Curr. Med. Chem. 2013, 20, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Ulyanova, V.; Ilinskaya, O.; Pleschka, S.; Shah Mahmud, R. A Novel Antiviral Strategy against MERS-CoV and HCoV-229E Using Binase to Target Viral Genome Replication. Bionanoscience 2017, 7, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Shah Mahmud, R.; Mostafa, A.; Müller, C.; Kanrai, P.; Ulyanova, V.; Sokurenko, Y.; Dzieciolowski, J.; Kuznetsova, I.; Ilinskaya, O.; Pleschka, S. Bacterial Ribonuclease Binase Exerts an Intra-Cellular Anti-Viral Mode of Action Targeting Viral RNAs in Influenza a Virus-Infected MDCK-II Cells. Virol. J. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah Mahmud, R.; Efimova, M.A.; Ulyanova, V.; Ravilov, R.K.; Shuralev, E.A.; Kolpakov, A.; Ilinskaya, O. Bacillus pumilus Ribonuclease Rescues Mice Infected by Double-Stranded RNA-Containing Reovirus Serotype 1. Virus Res. 2020, 286, 198086. [Google Scholar] [CrossRef]
- Makarov, A.A.; Ilinskaya, O.N. Cytotoxic Ribonucleases: Molecular Weapons and Their Targets. FEBS Lett. 2003, 540, 15–20. [Google Scholar] [CrossRef]
- Mitkevich, V.A.; Ilinskaya, O.N.; Makarov, A.A. Antitumor RNases: Killer’s Secrets. Cell Cycle 2015, 14, 931–932. [Google Scholar] [CrossRef]
- Mohamed, I.S.E.; Sen’kova, A.V.; Nadyrova, A.I.; Savin, I.A.; Markov, A.V.; Mitkevich, V.A.; Makarov, A.A.; Ilinskaya, O.N.; Mironova, N.L.; Zenkova, M.A. Antitumour Activity of the Ribonuclease Binase from Bacillus pumilus in the RLS40 Tumour Model Is Associated with the Reorganisation of the miRNA Network and Reversion of Cancer-Related Cascades to Normal Functioning. Biomolecules 2020, 10, 1509. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity: Miniperspective. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Dudkina, E.; Ulyanova, V.; Shah Mahmud, R.; Khodzhaeva, V.; Dao, L.; Vershinina, V.; Kolpakov, A.; Ilinskaya, O. Three-step Procedure for Preparation of Pure Bacillus altitudinis Ribonuclease. FEBS Open Bio 2016, 6, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, G.I.; Moiseyev, G.P.; Struminskaya, N.K.; Borzykh, O.A.; Kipenskaya, L.V.; Znamenskaya, L.V.; Leschinskaya, I.B.; Chernokalskaya, E.B.; Hartley, R.W. Mutational Analysis of the Active Site of RNase of Bacillus intermedius (BINASE). FEBS Lett. 1994, 354, 305–306. [Google Scholar] [CrossRef] [Green Version]
- Ilinskaya, O.; Karamova, N.; Ivanchenko, O.; Kipenskaya, L. SOS-Inducing Ability of Native and Mutant Microbial Ribonucleases. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 1996, 354, 203–209. [Google Scholar] [CrossRef]
- Kolpakov, A.I.; Il’inskaia, O.N. The optimization of a method for determining RNAse activity by using high-polymer RNA. Klin. Lab. Diagn. 1999, 5, 14–16. [Google Scholar]
- Sun, F.; Zong, W.; Liu, R.; Chai, J.; Liu, Y. Micro-Environmental Influences on the Fluorescence of Tryptophan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 142–145. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation–Release Performance of Biodegradable/Cytocompatible Agarose–Polyethylene Glycol–Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Singh Chandel, A.K.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually Crosslinked Injectable Hydrogels of Poly(Ethylene Glycol) and Poly[(2-Dimethylamino)Ethyl Methacrylate]-b-Poly(N-Isopropyl Acrylamide) as a Wound Healing Promoter. J. Mater. Chem. B 2017, 5, 4955–4965. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-Graft-Poly[(2-Dimethylamino)Ethyl Methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Thom, D.; Boyd, J. Chiroptical and Stoichiometric Evidence of a Specific, Primary Dimerisation Process in Alginate Gelation. Carbohydr. Res. 1978, 66, 145–154. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshetnyak, Y.K.; Burstein, E.A. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. II. The Statistical Proof of Discreteness of Tryptophan Classes in Proteins. Biophys. J. 2001, 81, 1710–1734. [Google Scholar] [CrossRef] [Green Version]
- Reshetnyak, Y.K.; Koshevnik, Y.; Burstein, E.A. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. III. Correlation between Fluorescence and Microenvironment Parameters of Individual Tryptophan Residues. Biophys. J. 2001, 81, 1735–1758. [Google Scholar] [CrossRef] [Green Version]
- Burstein, E.A.; Abornev, S.M.; Reshetnyak, Y.K. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. I. Decomposition Algorithms. Biophys. J. 2001, 81, 1699–1709. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Meng, L.; Lu, Q. Cell Behaviors on Polysaccharide-Wrapped Single-Wall Carbon Nanotubes: A Quantitative Study of the Surface Properties of Biomimetic Nanofibrous Scaffolds. ACS Nano 2009, 3, 3200–3206. [Google Scholar] [CrossRef]
- Heister, E.; Brunner, E.W.; Dieckmann, G.R.; Jurewicz, I.; Dalton, A.B. Are Carbon Nanotubes a Natural Solution? Applications in Biology and Medicine. ACS Appl. Mater. Interfaces 2013, 5, 1870–1891. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. PH-Sensitive Chitosan/Alginate Core-Shell Nanoparticles for Efficient and Safe Oral Insulin Delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Fenn, S.L.; Miao, T.; Scherrer, R.M.; Floreani, R.A. Dual-Cross-Linked Methacrylated Alginate Sub-Microspheres for Intracellular Chemotherapeutic Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17775–17783. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Huang, J. Study on the interaction of gelatin with transition metal ions. Acta Chim. Sin. 2001, 59, 1258–1264. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Matyash, M.; Despang, F.; Ikonomidou, C.; Gelinsky, M. Swelling and Mechanical Properties of Alginate Hydrogels with Respect to Promotion of Neural Growth. Tissue Eng. Part C Methods 2014, 20, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdown, A.B.G. 9. Metal Ions Affecting the Skin and Eyes. In Metal Ions in Life Sciences; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 187–246. ISBN 978-1-84973-091-4. [Google Scholar]
- Suzuki, Y.; Nishimura, Y.; Tanihara, M.; Suzuki, K.; Nakamura, T.; Shimizu, Y.; Yamawaki, Y.; Kakimaru, Y. Evaluation of a Novel Alginate Gel Dressing: Cytotoxicity to Fibroblastsin Vitro and Foreign-Body Reaction in Pig Skinin Vivo. J. Biomed. Mater. Res. 1998, 39, 317–322. [Google Scholar] [CrossRef]
- Mitkevich, V.A.; Makarov, A.A.; Ilinskaya, O.N. Cell Targets of Antitumor Ribonucleases. Mol. Biol. 2014, 48, 181–188. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Singh, I.; Dudkina, E.; Ulyanova, V.; Kayumov, A.; Barreto, G. Direct Inhibition of Oncogenic KRAS by Bacillus pumilus Ribonuclease (Binase). Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1559–1567. [Google Scholar] [CrossRef]







| Cation | Water Swelling (%) |
|---|---|
| Ca2+ | 287 |
| Mn2+ | 408 |
| Ni2+ | 432 |
| Cu2+ | 409 |
| Zn2+ | 250 |
| Ba2+ | 323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, L.R.; Zelenikhin, P.V.; Makarova, A.O.; Zueva, O.S.; Salnikov, V.V.; Zuev, Y.F.; Ilinskaya, O.N. Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase. Polymers 2022, 14, 2461. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122461
Bogdanova LR, Zelenikhin PV, Makarova AO, Zueva OS, Salnikov VV, Zuev YF, Ilinskaya ON. Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase. Polymers. 2022; 14(12):2461. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122461
Chicago/Turabian StyleBogdanova, Liliya R., Pavel V. Zelenikhin, Anastasiya O. Makarova, Olga S. Zueva, Vadim V. Salnikov, Yuriy F. Zuev, and Olga N. Ilinskaya. 2022. "Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase" Polymers 14, no. 12: 2461. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122461