Water Sorption and Mechanical Properties of Cellulosic Derivative Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Infrared Spectroscopy (FTIR)
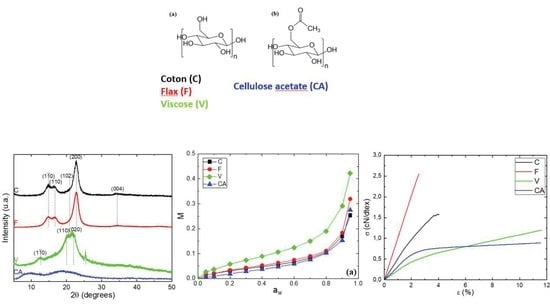
2.3. Wide-Angle X-ray Scattering (WAXS)
2.4. Vapor Water Sorption
2.5. Mechanical Properties
3. Analysis
4. Results and Discussion
4.1. Chemical Structure
4.2. Crystalline Morphology
4.3. Water Sorption
4.3.1. Sorption Isotherms
4.3.2. Mean Cluster Size (MCS)
4.4. Mechanical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolez, P.I.; Marsha, S.; McQueen, R.H. Fibers and Textiles for Personal Protective Equipment: Review of Recent Progress and Perspectives on Future Developments. Textiles 2022, 2, 349–381. [Google Scholar] [CrossRef]
- Gond, R.K.; Naik, T.P.; Gupta, M.K.; Singh, I. Development and characterisation of sugarcane bagasse nanocellulose/PLA composites. Mater. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awais, H.; Nawab, Y.; Amjad, A.; Anjang, A.; Akil, H.; Abidin, M.S.Z. Environmental benign natural fibre reinforced thermoplastic composites: A review. Compos. Part C Open Access 2021, 4, 100082. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Norton, A.; Newman, G. The water vapor sorption behavior of natural fibers. J. Appl. Polym. Sci. 2009, 112, 1524–1537. [Google Scholar] [CrossRef]
- Hsieh, Y.L. Chemical Structure and Properties of Cotton. In Cotton: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007; p. 528. [Google Scholar]
- Kabir, M.; Wang, H.; Lau, K.; Cardona, F. Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Kaushik, V.K.; Kumar, A.; Kalia, S. Effect of Mercerization and Benzoyl Peroxide Treatment on Morphology, Thermal Stability and Crystallinity of Sisal Fibers. Int. J. Text. Sci. 2012, 1, 101–105. [Google Scholar] [CrossRef]
- Meredith, R. 10—The Tensile Behaviour of Raw Cotton and Other Textile Fibres. J. Text. Inst. Trans. 1945, 36, T107–T130. [Google Scholar] [CrossRef]
- Malm, C.J.; Tanghe, L.J.; Laird, B.C. Preparation of Cellulose Acetate—Action of Sulfuric Acid. Ind. Eng. Chem. 1946, 38, 77–82. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2014, 49, 2362–2371. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Okubayashi, S.; Griesser, U.J.; Bechtold, T. Moisture sorption/desorption behavior of various manmade cellulosic fibers. J. Appl. Polym. Sci. 2005, 97, 1621–1625. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley and Sons: Hoboken, NJ, USA, 2006; ISBN 9780470021729. [Google Scholar]
- Jonquières, A.; Fane, A. Modified BET Models for Modeling Water Vapor Sorption in Hydrophilic Glassy Polymers and Systems Deviating Strongly from Ideality. J. Appl. Polym. Sci. 1998, 67, 1415–1430. [Google Scholar] [CrossRef]
- Leung, H. Water Activity and Other Colligative Properties of Foods. In Proceedings of the ASAE Annual Meeting, Chicago, IL, USA, 14–15 October 1983; p. 6508. [Google Scholar]
- Labuza, T.P. The Effect of Water Activity on Reaction Kinetics of Food Deterioration. Food Technol. 1980, 34, 36–41,59. [Google Scholar]
- Chirife, J.; Fontan, C.F.; Benmergui, E. The prediction of water activity in aqueous solutions in connection with intermediate moisture foods IV. aW Prediction in aqueous non electrolyte solutions. Int. J. Food Sci. Technol. 1980, 15, 59–70. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a Semi-Empirical Four Parameter General Model for Sigmoid Moisture Sorption Isotherms. J. Food Process Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
- Smith, S.E. The Sorption of Water Vapor by High Polymers. J. Am. Chem. Soc. 1947, 69, 646–651. [Google Scholar] [CrossRef]
- Iglesias, H.A.; Chirife, J. A model for describing the water sorption behavior of foods. J. Food Sci. 1976, 41, 984–992. [Google Scholar] [CrossRef]
- Guggenheim, E.A. Applications of Statistical Mechanics; Clarendon Press: Oxford, UK, 1966. [Google Scholar]
- Park, G.S. Transport Principles—Solution, Diffusion and Permeation in Polymer Membranes. In Synthetic Membranes: Science, Engineering and Applications; Springer: Dordrecht, The Netherlands, 1986; pp. 57–107. [Google Scholar] [CrossRef]
- Filho, G.R.; Monteiro, D.S.; Meireles, C.D.S.; de Assunção, R.M.N.; Cerqueira, D.; Barud, H.S.; Ribeiro, S.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Bledzki, A.K.K.; Gassan, J. Composites Reinforced with Cellulose. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Lilholt, H.; Lawther, J.M. Natural Organic Fibers. In Comprehensive Composite Materials; Pergamon: Oxford, UK, 2000; pp. 303–325. [Google Scholar] [CrossRef]
- Olesen, P.O.; Placket, D.V. Perspectives on the Performance of Natural Plant Fibres. In Proceedings of the Natural Fibres Performance Forum, Copenhagen, Denmark, 27 May 1999; p. 7. [Google Scholar]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Farahnaky, A.; Ansari, S.; Majzoobi, M. Effect of glycerol on the moisture sorption isotherms of figs. J. Food Eng. 2009, 93, 468–473. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Water sorption isotherms of starch powders: Part 1: Mathematical description of experimental data. J. Food Eng. 2004, 61, 297–307. [Google Scholar] [CrossRef]
- Lomauro, C.J.; Bakshi, A.S.; Labuza, T.P. Evaluation of Food Moisture Sorption Isotherm Equations 1. Fruit, Vegetable and Meat-Products. Lebensm. Wissenchaft Technol. 1985, 18, 111–117. [Google Scholar]
- Zimm, B.H.; Lundberg, J.L. Sorption of Vapors by High Polymers. J. Phys. Chem. 1956, 60, 425–428. [Google Scholar] [CrossRef]
- Lundberg, J.L. Molecular clustering and segregation in sorption systems. Pure Appl. Chem. 1972, 31, 261–282. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. Characterization and modeling of the moisture diffusion behavior of natural fibers. J. Appl. Polym. Sci. 2013, 130, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Alix, S.; Philippe, E.; Bessadok, A.; Lebrun, L.; Morvan, C.; Marais, S. Effect of chemical treatments on water sorption and mechanical properties of flax fibres. Bioresour. Technol. 2009, 100, 4742–4749. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and characterization of cellulose acetate from rice husk: Eco-friendly condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Kafle, K.; Greeson, K.; Lee, C.; Kim, S.H. Cellulose polymorphs and physical properties of cotton fabrics processed with commercial textile mills for mercerization and liquid ammonia treatments. Text. Res. J. 2014, 84, 1692–1699. [Google Scholar] [CrossRef]
- Karacan, I.; Soy, T. Investigation of structural transformations taking place during oxidative stabilization of viscose rayon precursor fibers prior to carbonization and activation. J. Mol. Struct. 2013, 1041, 29–38. [Google Scholar] [CrossRef]
- Kale, R.D.; Gorade, V.G.; Parmaj, O. Novel Sericin/Viscose Rayon-Based Biocomposite: Preparation and Characterization. J. Nat. Fibers 2020, 17, 532–541. [Google Scholar] [CrossRef]
- Hindeleh, A.; Johnson, D. Crystallinity and crystallite size measurement in cellulose fibres: 2. Viscose rayon. Polymer 1974, 15, 697–705. [Google Scholar] [CrossRef]
- Ibbett, R.N.; Domvoglou, D.; Phillips, D.A.S. The hydrolysis and recrystallisation of lyocell and comparative cellulosic fibres in solutions of mineral acid. Cellulose 2008, 15, 241–254. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Deus, C.; Friebolin, H.; Siefert, E. Partiell acetylierte cellulose—Synthese und bestimmung der substituentenverteilung mit hilfe der 1H NMR-spektroskopie. Makromol. Chem. 1991, 192, 75–83. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled heterogeneous modification of cellulose fibers with fatty acids: Effect of reaction conditions on the extent of esterification and fiber properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Wu, S.; Qin, X.; Li, M. The structure and properties of cellulose acetate materials: A comparative study on electrospun membranes and casted films. J. Ind. Text. 2014, 44, 85–98. [Google Scholar] [CrossRef]
- Mikhalovska, L.I.; Gun’Ko, V.M.; Rugal, A.A.; Oranska, O.I.; Gornikov, Y.I.; Morvan, C.; Follain, N.; Domas, C.; Pakhlov, E.M.; Mikhalovsky, S.V. Cottonised flax fibres vs. cotton fibres: Structural, textural and adsorption characteristics. RSC Adv. 2012, 2, 2032–2042. [Google Scholar] [CrossRef]
- Yueping, W.; Ge, W.; Haitao, C.; Genlin, T.; Zheng, L.; Feng, X.Q.; Xiangqi, Z.; Xiaojun, H.; Xushan, G. Structures of Bamboo Fiber for Textiles. Text. Res. J. 2010, 80, 334–343. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Xu, Q.; Wang, H. Solvent-free acetylation of bacterial cellulose under moderate conditions. Carbohydr. Polym. 2011, 83, 1575–1581. [Google Scholar] [CrossRef]
- Okubayashi, S.; Griesser, U.; Bechtold, T. A kinetic study of moisture sorption and desorption on lyocell fibers. Carbohydr. Polym. 2004, 58, 293–299. [Google Scholar] [CrossRef]
- Mihranyan, A.; Llagostera, A.P.; Karmhag, R.; Strømme, M.; Ek, R. Moisture sorption by cellulose powders of varying crystallinity. Int. J. Pharm. 2004, 269, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sabard, M.; Gouanvé, F.; Espuche, E.; Fulchiron, R.; Seytre, G.; Fillot, L.-A.; Trouillet-Fonti, L. Influence of film processing conditions on the morphology of polyamide 6: Consequences on water and ethanol sorption properties. J. Membr. Sci. 2012, 415–416, 670–680. [Google Scholar] [CrossRef]
- Ormondroyd, G.A.; Curling, S.F.; Mansour, E.; Hill, C.A.S. The water vapour sorption characteristics and kinetics of different wool types. J. Text. Inst. 2017, 108, 1198–1210. [Google Scholar] [CrossRef]
- Masclaux, C.; Gouanvé, F.; Espuche, E. Experimental and modelling studies of transport in starch nanocomposite films as affected by relative humidity. J. Membr. Sci. 2010, 363, 221–231. [Google Scholar] [CrossRef]
- Cunha, A.G.; Zhou, Q.; Larsson, P.T.; Berglund, L.A. Topochemical acetylation of cellulose nanopaper structures for biocomposites: Mechanisms for reduced water vapour sorption. Cellulose 2014, 21, 2773–2787. [Google Scholar] [CrossRef] [Green Version]
- Gouanvé, F.; Marais, S.; Bessadok, A.; Langevin, D.; Morvan, C.; Métayer, M. Study of water sorption in modified flax fibers. J. Appl. Polym. Sci. 2006, 101, 4281–4289. [Google Scholar] [CrossRef]
- Bessadok, A.; Langevin, D.; Gouanvé, F.; Chappey, C.; Roudesli, S.; Marais, S. Study of water sorption on modified Agave fibres. Carbohydr. Polym. 2009, 76, 74–85. [Google Scholar] [CrossRef]
- Himmel, S.; Mai, C. Water vapour sorption of wood modified by acetylation and formalization—Analysed by a sorption kinetics model and thermodynamic considerations. Holzforschung 2016, 70, 203–213. [Google Scholar] [CrossRef]
- Davis, E.M.; Elabd, Y.A. Water Clustering in Glassy Polymers. J. Phys. Chem. B 2013, 117, 10629–10640. [Google Scholar] [CrossRef] [PubMed]
- Baley, C. Analysis of the flax fibres tensile behaviour and analysis of the tensile stiffness increase. Compos. Part A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Charlet, K.; Baley, C.; Morvan, C.; Jernot, J.; Gomina, M.; Bréard, J. Characteristics of Hermès flax fibres as a function of their location in the stem and properties of the derived unidirectional composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1912–1921. [Google Scholar] [CrossRef]
- Placet, V.; Cissé, O.; Boubakar, M.L. Nonlinear tensile behaviour of elementary hemp fibres. Part I: Investigation of the possible origins using repeated progressive loading with in situ microscopic observations. Compos. Part A Appl. Sci. Manuf. 2014, 56, 319–327. [Google Scholar] [CrossRef] [Green Version]










| Material | Mass Composition (%) | Yarn Count (dtex) | DS | Ref. | |||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Pectin | Lignin | ||||
| C | 82–99 | 3–6 | 0–5.7 | - | 100 | [26,27,28,29] | |
| F | 64–85 | 10–20.6 | 2.3–12 | 0–5 | 400 | ||
| V | 100 | - | - | - | 250 | - | |
| CA | 100 | - | - | - | 80 | 2.1 1 | [25] |
| Material | |
|---|---|
| C | 61 ± 3 |
| F | 67 ± 2 |
| V | 32 ± 1 |
| CA | <10 |
| Material | |||||||
|---|---|---|---|---|---|---|---|
| C | S | 0.0100 | 60 | 0.090 | 0.290 | 13 | 8.3 |
| D | 0.0100 | 60 | 0.120 | 0.260 | 14 | 6.3 | |
| F | S | 0.0102 | 81 | 0.107 | 0.516 | 18 | 2.6 |
| D | 0.0106 | 70 | 0.126 | 0.504 | 18 | 3.2 | |
| V | S | 0.0311 | 35 | 0.152 | 0.387 | 10 | 2.1 |
| D | 0.0317 | 38 | 0.202 | 0.377 | 12 | 2.3 | |
| CA | S | 0.0000 | 0 | 0.104 | 0.456 | 19 | 8.0 |
| D | 0.0000 | 0 | 0.125 | 0.447 | 18 | 9.6 |
| Material | (cN/dtex) | (cN/dtex) | (%) |
|---|---|---|---|
| C | 53 ± 3 | 1.6 ± 0.2 | 4.4 ± 0.4 |
| F | 120 ± 10 | 3.2 ± 0.6 | 2.7 ± 0.5 |
| V | 20 ± 2 | 1.1 ± 0.2 | 13.1 ± 1.8 |
| CA | 36 ± 3 | 0.9 ± 0.2 | 9.0 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, M.; Fulchiron, R.; Gouanvé, F. Water Sorption and Mechanical Properties of Cellulosic Derivative Fibers. Polymers 2022, 14, 2836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142836
Simon M, Fulchiron R, Gouanvé F. Water Sorption and Mechanical Properties of Cellulosic Derivative Fibers. Polymers. 2022; 14(14):2836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142836
Chicago/Turabian StyleSimon, Mathilde, René Fulchiron, and Fabrice Gouanvé. 2022. "Water Sorption and Mechanical Properties of Cellulosic Derivative Fibers" Polymers 14, no. 14: 2836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142836