1. Introduction
Starting at subzero temperatures is one of the main hurdles in commercializing polymer electrolyte membrane fuel cell (PEMFC). A successful cold start would require a rapid temperature rise to avoid ice formation and water build-up in the porous electrode layers. A well-controlled cold start can help mitigate or even eliminate the potential damage caused by freeze/thaw or cold-start cycles.
To reduce this possible damage and enable a successful cold start, researchers have attempted to optimize start-up strategies by removing as much water as possible by gas purging [
1,
2] during cell shutdown, by preheating the fuel cell to increase its temperature as quickly as possible [
3,
4,
5,
6], by utilizing different current loading modes during the start-up process [
7]. Meanwhile, people also tried to improve the cold start performance of fuel cells by considering the effects of end plate [
8], flow-field structure [
9] and the membrane electrode assembly (MEA) material components [
10]. So far, numerous experimental and modeling studies have been conducted to investigate the reasons for the damage caused to the MEA, such as cracks and pinholes on the membrane [
11,
12], local catalyst cracks [
13], interfacial catalyst layer (CL)/membrane and CL/gas diffusion layer (GDL) delamination [
14,
15], loss of electrochemical surface area [
16], and variation in the hydrophobicity of the GDL [
17,
18].
Almost all of the above-mentioned damages to the MEA are related to water in the fuel cell and are caused by freeze/thaw cycles or the cold start operation; therefore, researchers have attempted to study the water phase states, phase changes [
19,
20,
21,
22], water transport models [
23,
24,
25], liquid and ice distribution [
26,
27], and other behaviors of water in fuel cells at subzero temperatures.
Interestingly, one of the states of water in the PEMFC at a subzero temperature (supercooled liquid) is attracting increasing attention from researchers. S.H. Ge et al. [
28,
29] developed a transparent PEFC to study liquid water conversion and ice formation during start-up from subzero temperatures; they used a silver mesh as the cathode GDL to directly observe the phase change and water transport on the surface of the CL. It was found that at a current density of 0.02 A/cm
2 and start-up temperature of −5 °C, water in the cathode CL existed in the solid and gaseous phases. However, when start-up temperatures higher than −3 °C, water droplets were found on the CL surface, and the cold start operation was significantly prolonged. Based on this, they suggested that the freezing-point depression of water in the cathode CL is not greater than 1 ± 0.5 °C and that it plays a negligible role in the cold start theory and its applications. J. B. Ryan et al. [
30,
31] considered supercooled water in their theoretical modeling and defined freezing-point depression as the difference between the freezing point in a porous material and the normal freezing point of water; generally the freezing-point depression of water is only about 1 ± 0.5 °C. C.W. Park et al. [
20] reported their work on the supercooling release of micro-sized water droplets on GDL surfaces with cooling. It was found that the average supercooling degrees of water droplets decreased as the size of water droplets increased from 6 μL to 15 and 30 μL on the hydrophobic GDL surface, while they increased from 6.9 K to 7.5 and 10.1 K as the PTFE coating rate of the GDL increased from 0% to 40% and 60% PTFE contents, respectively. Notably, the water on the GDL surface could remain in the liquid phase for several minutes at a supercooling degree of 7–10 °C, depending on properties, such as the PTFE content, size of the water droplet, among others. P Oberholzer et al. [
32] used high-resolution dynamic in-plane neutron imaging to investigate the mechanism of water accumulation during a PEFC cold start. In their work, a condensed water phase was observed to accumulate not only in the MEA but also in the cathode GDL at −15 °C and even in the cathode gas channels at −10 °C. Y. Ishikawa et al. [
19] tested a fuel cell at −10 °C; they measured the temperature of the water on the CL using thermal imaging and observed the behavior of the water using a microscope under appropriate illumination. They found that the generated water was in a supercooled state, and the diameter of the water droplets was approximately 10 μm when generated. Subsequently, the size of the droplets increased considerably, absorbing the smaller water droplets in the vicinity and, consequently, becoming larger and fewer. Furthermore, the supercooled state was maintained while these physical movements occurred. In addition, the droplets froze when they expanded to the diameter of approximately 100 μm, after which the temperature rose significantly. Generally, when water in a supercooled state begins to freeze, it emits heat of solidification, and the temperature rises to 0 °C. Y. Ishikawa et al. [
33] also developed a system capable of acquiring cross-sectional visible and infrared images inside the fuel cell, and they used this system to observe the supercooled water and freezing phenomena. They found that supercooled water was generated on the GDL surface, and water froze at the interface between the GDL and MEA. Using infrared radiation imaging, it was clarified that the heat of solidification disperses at the GDL/MEA interface the moment the cell performance drops. The ice formation at the GDL/MEA interface causes air gas stoppage and consequently affects cell performance.
Recently, Y. Ishikawa et al. [
34] theoretically analyzed the supercooled states of water generated below the freezing point in a PEFC and demonstrated, experimentally, that inside the CL, water can be present in the liquid state for 340 s and 70 s when the supercooling degrees are 10 °C and 20 °C, respectively. Based on the heterogeneous nucleation theory and by considering the surface wettability of the porous media in the cells, they developed a theoretical model to predict the release of supercooled states. The model successfully reproduced the supercooled state in the cell, specifically its release time, and quantitatively clarified the effect of the pore diameter and wettability on the supercooled states. T.J. Dursch et al. [
35,
36,
37] experimentally studied the ice crystallization kinetics (ICK) of water in the GDL and CL at subzero temperatures, including the effects of the rate of temperature decrease and supercooling degree on the induction time for ice crystallization (i.e., survival time for liquid water), and the crystallization rate. Therefore, water can be a supercooled liquid in the PEMFC when it is in a subzero temperature environment, although the freezing-point depression varies in a wide range; the possible reason for this is the absence of ice nuclei. Water can be maintained in a metastable liquid state in the temperature range of −42–0 °C, and its stability depends on the probability of formation and growth of ice nuclei. The presence of the liquid state and its survival time may impact water’s movement, phase transfer, and, consequently, the ice distribution inside the cell, as well as the preheating methods and optimal control strategy for the cold start. To the best of our knowledge, only a few studies have focused on this topic, among which T.J. Dursch et al. mentioned a 1D isothermal PEMFC cold-start model in their experimental research work about the ICK in CL [
37]; in the model, the ICK was considered, but both the model and the results were introduced very simply. L Yao et al. numerically investigated the cold-start behavior of PEMFCs in the presence of supercooled water [
38], assuming that when the freezing probability of the supercooled liquid reaches one, the liquid water inside the cell would freeze simultaneously, and no liquid water will exist in the cell.
In the early work [
15], with a 2D model, we studied the mechanical response induced by the ice formation in the MEA during a failed start up procedure of fuel cell, and the stress and strain distribution and evolution were studied; the MEA may be damaged by the stress. In this study, a 2D transient multi-physic model was developed to simulate the cold-start processes in a PEMFC; the phase change between vapor water, liquid water and ice in the CLs, MPLs and GDLs was included; particularly, the ICK was considered when super cold liquid water changes into ice. The factors of different operating conditions and MEA wettability were investigated. The following sections contain a brief introduction to the characteristics of ICK, model assumptions, details of the complete mathematical model, boundary conditions and numerical procedures, results and discussion, and finally conclusions.
2. Ice Crystallization Kinetics
According to the heterogeneous nucleation theory [
39], critical clusters may form in supercooled water at a certain supercooling degree. The production rate of such clusters,
J (nuclei cm
−3s
−1), can be expressed by
or
and
where
nL is the number density of water molecules,
k is the Boltzmann constant,
T is the temperature (K),
h is the Planck constant, Δ
g is the activation energy of water molecules, Δ
G* is the Gibbs-free energy of critical nucleus formation, σ is the surface tension of the cluster and water,
Te is the melting temperature,
ρ is the mass density of water,
hcond is the latent heat of condensation, Δ
T is the supercooling degree, and
f(
θ) is the energy barrier coefficient of nucleation.
A supercooled state is released when the total number of critical clusters reaches a threshold value of 1. The total number of critical clusters can be calculated by integrating the product of the water volume,
V0, and the critical cluster nucleation rate [
34].
The time from the moment liquid water is produced to the moment it is released is called the induction time,
τi, expressed by the equation below, where
J and
V are constants:
After the release, the liquid-to-ice conversion rate, i.e., the crystallization rate of water,
, follows [
36,
37,
40]:
where
t is the time elapsed after the release,
is the ice volume fraction:
and
q(
T) is the crystallization rate constant (s
−2.5) under a certain contact angle,
θ, and supercooling degree, Δ
T.
Therefore, the liquid converts to ice at a limited rate, which differs from the instantaneous phase change based on thermodynamics [
23,
24,
38,
41].
Theoretically, using the constants and parameters in
Table 1, the relationship between the nuclei production rate, induction time, and ice crystallization rate with respect to the supercooling degree and contact angle can be computed theoretically, as shown in
Figure 1.
Figure 1a,b show that, at a given supercooling degree, Δ
T, the ice crystal nucleus production rate,
J, decreases and the induction time,
, increases with the increase in the contact angle,
θ; under a given
θ,
J decreases and
increases as Δ
T decreases.
Figure 1c shows that after the release, the ice volume fraction,
, increases continuously until 100% is attained; however, the ice formation rate,
Ri(
t;
T), is relatively low at the beginning and ending periods, while it is high during the middle period. Meanwhile, at a certain time and contact angle, their values increase with the supercooling degree. An implication of these trends is that the ice formation in the MEA would be suppressed at high contact angles (or on a more hydrophobic surface) [
42,
43]. Naturally, such ICK will affect the ice formation and its distribution inside the MEA during a cold start.
Contrarily, due to the complexity of the microstructure and the material ingredients of the MEA, it is difficult to calculate the theoretical values of
J,
τi, and
φ even at a given supercooling degree, Δ
T. Therefore, in this study, the measured data from Refs. [
35,
36,
37] will be used to calculate
, which is related to coefficients
A and
B in Equation (2). Meanwhile, T.J. Dursch et al. verified that for some different CL and GDL materials, ln
J versus
T−1(Δ
T)
−2 produces a straight line with an intercept of ln
A and slope of −
B, which agrees with Equation (2) [
37].
5. Conclusions
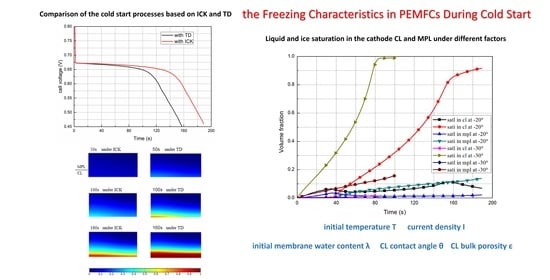
In this study, a 2D transient multi-physic model was developed to simulate the cold start processes in a PEM fuel cell, the ice crystallization kinetics was considered when supercooled liquid water changes into ice, other phase change between different water states inside the MEA was included also. The model was verified by the general agreement between the predicted data and the experimental data. Thereafter, the results of the two models assuming TD and assuming ICK were analyzed and compared, the effects on the cold start processes of start temperature, current density, the wettability of the CL, and the porosity of CL were investigated. The following conclusions can be drawn.
When start temperature is −20 °C or higher, compared with models assuming TD, ice formation is delayed and the formation rate is decreased for the model assuming ICK, and more supercooled liquid water permeates from CL into MPL and GDL. Therefore, the fuel-cell performance with ICK is better, and the cold start process can be sustained for a longer time. The effect of ice crystallization kinetics is negligible when the fuel cell is started from −30 °C and below; in this case, for achieving a successfully cold start and reducing the possible damage to the MEA during the icing process, it is recommended to preheat the fuel cells to around −15 °C firstly. For a cold start with lower current density, less water is produced during the start process, and the induction time will be increased; during the same period, the amount of ice accumulated in the CL is reduced, and the cold start process can be sustained for a longer time. When the CL has a relatively large contact angle, it is more hydrophobic, and the induction time increases; consequently, more liquid water is accumulated and permeated into MPL and even GDL. As a result, the fuel-cell running time (from the beginning to the failure moment) increases.
According to the findings of this study, we propose an optimal operation control strategy for the fuel cell cold start process as follows: when the fuel cell is started from −30 °C and below, it is recommended to preheat the fuel cells to around −15 °C firstly. Then a lower current density is applied to hydrate the membrane, followed by a current density as large as possible to generate more heat to increase the temperature of the cell quickly, together with a larger air flow rate to blow the liquid water out of the cell as possible. These findings are beneficial for PEMFCs to start up from subzero temperature.