Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Screening of EPS-Producing Capacity
2.2. Growth of Bacterial Strain and EPS Production
2.3. Extraction and Purification of EPS
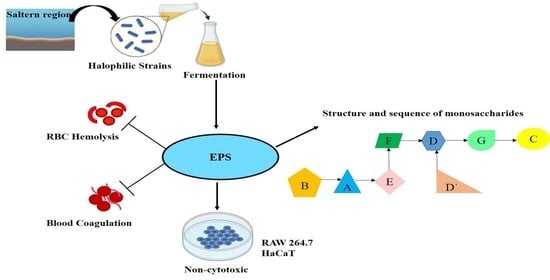
Characterization of EPS
2.4. Scanning Electron Microscopy, Energy Dispersive X-ray, and Atomic Force Microscopy
2.5. Zeta Potential
2.6. High-Performance Liquid Chromatography
2.7. Fourier Transform-Infrared Spectroscopy (FT-IR)
2.8. Molecular Weight Determination
2.9. Nuclear Magnetic Resonance Spectroscopy
2.10. Thermogravimetric Analysis
2.11. X-ray Diffraction Analysis
2.12. Water Solubility Value, Water-Holding Capacity, and Emulsifying Activity
2.12.1. Water Solubility Value
2.12.2. Water-Holding Capacity
2.12.3. Emulsifying Activity
2.13. Hemocompatibility and Erythrocyte Membrane stabilization Activity
2.13.1. Preparation of Suspension (10% v/v) of Human Red Blood Cell
2.13.2. Hemolytic Activity Assay
2.13.3. Hypotonic Solution-Induced Hemolysis
2.13.4. Heat-Induced Hemolysis
2.14. Anticoagulant Activity
2.15. In Vitro Cytocompatibility
Cell Viability Assay
2.16. Statistical Analysis
3. Results and Discussion
3.1. Bacterial Strain and EPS-Producing Capacity
3.2. Bacterial Growth and EPS Production
3.3. Purification
3.4. Microstructure and Elemental Analysis of EPS
3.5. Zeta Potential
3.6. HPLC Analysis
3.7. Fourier Transform-Infrared Spectroscopy (FT-IR)
3.8. Molecular Weight
3.9. NMR Analysis
3.10. Thermogravimetric Analysis
3.11. X-ray Diffraction Analysis
3.12. Water Solubility, Water-Holding Capacity, and Emulsifying Activity
3.13. Hemocompatibility and Erythrocyte Membrane Stabilization Activity of EPS
3.14. Anticoagulant Activity of EPS
3.15. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. Nat. Synth. Biomed. Polym. 2014, 67–89. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B. Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Gupta, A.; Agrawal, G. Recent Advances in Polysaccharides Based Biomaterials for Drug Delivery and Tissue Engineering Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A Critical Review of Extracellular Polymeric Substances (EPSs) in Membrane Bioreactors: Characteristics, Roles in Membrane Fouling and Control Strategies. J. Memb. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.-G.; Lee, K.H.; Chang, J.Y.; Kim, J.-C.; Park, H.W. Production, Characterization, and Antioxidant Activities of an Exopolysaccharide Extracted from Spent Media Wastewater after Leuconostoc mesenteroides WiKim32 Fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural Features of Microbial Exopolysaccharides in Relation to Their Antioxidant Activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: A Review and New Perspectives on Engineering Strategies and Applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of Polymer-Based Biomaterials and Medical Devices—Regulations, in Vitro Screening and Risk-Management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Komeri, R.; Kasoju, N.; Anil Kumar, P.R. In Vitro Cytotoxicity and Cytocompatibility Assays for Biomaterial Testing under Regulatory Platform. Biomed. Prod. Mater. Eval. Stand. Ethics 2022, 329–353. [Google Scholar] [CrossRef]
- Corral, P.; Amoozegar, M.A.; Ventosa, A. Halophiles and Their Biomolecules: Recent Advances and Future Applications in Biomedicine. Mar. Drugs 2020, 18, 33. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, Adaptation, and Their Role in Decontamination of Hypersaline Environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef]
- Singh, S.; Sran, K.S.; Pinnaka, A.K.; Roy Choudhury, A. Purification, Characterization and Functional Properties of Exopolysaccharide from a Novel Halophilic Natronotalea sambharensis Sp. Nov. Int. J. Biol. Macromol. 2019, 136, 547–558. [Google Scholar] [CrossRef]
- Shivanand, P.; Jayaraman, G. Production of Extracellular Protease from Halotolerant Bacterium, Bacillus aquimaris Strain VITP4 Isolated from Kumta Coast. Process Biochem. 2009, 44, 1088–1094. [Google Scholar] [CrossRef]
- Devi Rajeswari, V.; Jayaraman, G.; Rameshpathy, M.; Sridharan, T.B. Production and Characterization of Extracellular Protease from Halotolerant Bacterium Virgibacillus dokdonesis VITP14. Res. J. Biotechnol. 2012, 7, 38–42. [Google Scholar]
- Sahana, T.G.; Rekha, P.D. A Novel Exopolysaccharide from Marine Bacterium Pantoea Sp. YU16-S3 Accelerates Cutaneous Wound Healing through Wnt/β-Catenin Pathway. Carbohydr. Polym. 2020, 238, 116191. [Google Scholar] [CrossRef]
- Peng, Y.; Han, B.; Liu, W.; Zhou, R. Deproteinization and Structural Characterization of Bioactive Exopolysaccharides from Ganoderma Sinense Mycelium. Sep. Sci. Technol. 2016, 51, 359–369. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide Composition Influence and Immunomodulatory Effects of Probiotic Exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pan, Z.; Zeng, B.; Yu, G.; Teng, J.; Zhang, H.; Shen, L.; Yang, L.; Lin, H. Mechanistic Insights into Ca-Alginate Gel-Associated Membrane Fouling Affected by Ethylene Diamine Tetraacetic Acid (EDTA). Sci. Total Environ. 2022, 842, 156912. [Google Scholar] [CrossRef]
- Zeng, B.; Pan, Z.; Shen, L.; Zhao, D.; Teng, J.; Hong, H.; Lin, H. Effects of Polysaccharides’ Molecular Structure on Membrane Fouling and the Related Mechanisms. Sci. Total Environ. 2022, 836, 155579. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Y.; Shen, L.; Li, R.; Lin, H. Preparation of Nickel@polyvinyl Alcohol (PVA) Conductive Membranes to Couple a Novel Electrocoagulation-Membrane Separation System for Efficient Oil-Water Separation. J. Memb. Sci. 2022, 653, 120541. [Google Scholar] [CrossRef]
- Liu, J.; Shen, L.; Lin, H.; Huang, Z.; Hong, H.; Chen, C. Preparation of Ni@UiO-66 Incorporated Polyethersulfone (PES) Membrane by Magnetic Field Assisted Strategy to Improve Permeability and Photocatalytic Self-Cleaning Ability. J. Colloid Interface Sci. 2022, 618, 483–495. [Google Scholar] [CrossRef]
- Chen, B.; Hu, X.; Wang, J.; Li, R.; Shen, L.; Xu, Y.; Zhang, M.; Hong, H.; Lin, H. Novel Catalytic Self-Cleaning Membrane with Peroxymonosulfate Activation for Dual-Function Wastewater Purification: Performance and Mechanism. J. Clean. Prod. 2022, 355, 131858. [Google Scholar] [CrossRef]
- Pan, Z.; Zeng, B.; Lin, H.; Teng, J.; Zhang, H.; Hong, H.; Zhang, M. Fundamental Thermodynamic Mechanisms of Membrane Fouling Caused by Transparent Exopolymer Particles (TEP) in Water Treatment. Sci. Total Environ. 2022, 820, 153252. [Google Scholar] [CrossRef]
- Gangalla, R.; Gattu, S.; Palaniappan, S.; Ahamed, M.; Macha, B.; Thampu, R.K.; Fais, A.; Cincotti, A.; Gatto, G.; Dama, M.; et al. Structural Characterisation and Assessment of the Novel Bacillus amyloliquefaciens RK3 Exopolysaccharide on the Improvement of Cognitive Function in Alzheimer’s Disease Mice. Polymers 2021, 13, 2842. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z. Separation and Quantification of Component Monosaccharides of the Tea Polysaccharides from Gynostemma Pentaphyllum by HPLC with Indirect UV Detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Insulkar, P.; Kerkar, S.; Lele, S.S. Purification and Structural-Functional Characterization of an Exopolysaccharide from Bacillus licheniformis PASS26 with in-Vitro Antitumor and Wound Healing Activities. Int. J. Biol. Macromol. 2018, 120, 1441–1450. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and Characterization of Exopolysaccharide Produced by Weissella cibaria YB-1 from Pickle Chinese Cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, X.; Wang, H.; Peng, B.; Tian, Y. Structural Characterization and Functional Evaluation of a Novel Exopolysaccharide from the Moderate Halophile Gracilibacillus Sp. SCU50. Int. J. Biol. Macromol. 2020, 154, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Boujida, N.; Palau, M.; Charfi, S.; El Moussaoui, N.; Manresa, A.; Miñana-Galbis, D.; Skali Senhaji, N.; Abrini, J. Isolation and Characterization of Halophilic Bacteria Producing Exopolymers with Emulsifying and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2018, 16, 631–637. [Google Scholar] [CrossRef]
- Medlej, M.K.; Batoul, C.; Olleik, H.; Li, S.; Hijazi, A.; Nasser, G.; Maresca, M.; Pochat-Bohatier, C. Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon. Antioxidants 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Abbou, A.; Kadri, N.; Debbache, N.; Dairi, S.; Remini, H.; Dahmoune, F.; Berkani, F.; Adel, K.; Belbahi, A.; Madani, K. Effect of Precipitation Solvent on Some Biological Activities of Polysaccharides from Pinus halepensis Mill. Seeds. Int. J. Biol. Macromol. 2019, 141, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Vidhyalakshmi, R.; Valli Nachiyar, C.; Narendra Kumar, G.; Sunkar, S. Bacillus circulans Exopolysaccharide: Production, Characterization and Bioactivities. Int. J. Biol. Macromol. 2016, 87, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Wang, L.; Yu, Q.; Ai, C.; Fu, Y.; Yan, C.; Wen, C.; Zhu, Z. Structural Characterization and Anticoagulant Activity of Two Polysaccharides from Patinopecten yessoensis viscera. Int. J. Biol. Macromol. 2019, 136, 579–585. [Google Scholar] [CrossRef]
- He, C.; Lin, H.Y.; Wang, C.C.; Zhang, M.; Lin, Y.Y.; Huang, F.Y.; Lin, Y.Z.; Tan, G.H. Exopolysaccharide from Paecilomyces lilacinus Modulates Macrophage Activities through the TLR4/NF-κB/MAPK Pathway. Mol. Med. Rep. 2019, 20, 4943–4952. [Google Scholar] [CrossRef]
- Joghee, N.N.; Jayaraman, G. Metabolomic Characterization of Halophilic Bacterial Isolates Reveals Strains Synthesizing Rare Diaminoacids under Salt Stress. Biochimie 2014, 102, 102–111. [Google Scholar] [CrossRef]
- Joghee, N.N.; Jayaraman, G. Biochemical Changes Induced by Salt Stress in Halotolerant Bacterial Isolates Are Media Dependent as Well as Species Specific. Prep. Biochem Biotechnol. 2016, 46, 8–14. [Google Scholar] [CrossRef]
- Mudryk, Z.; Donderski, W. Effect of Sodium Chloride on the Metabolic Activity of Halophilic Bacteria Isolated from the Lake Gardno Estuary. Estuaries 1991, 14, 495–498. [Google Scholar] [CrossRef]
- Hereher, F.; ElFallal, A.; Abou-Dobara, M.; Toson, E.; Abdelaziz, M.M. Cultural Optimization of a New Exopolysaccharide Producer “Micrococcus roseus”. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 632–639. [Google Scholar] [CrossRef]
- Silvi, S.; Barghini, P.; Aquilanti, A.; Juarez-Jimenez, B.; Fenice, M. Physiologic and Metabolic Characterization of a New Marine Isolate (BM39) of Pantoea Sp. Producing High Levels of Exopolysaccharide. Microb. Cell Fact. 2013, 12, 10. [Google Scholar] [CrossRef]
- Abdelnasser, S.M.; Yahya, S.M.M.; Mohamed, W.F.; Asker, M.M.S.; Abu Shady, H.M.; Mahmoud, M.G.; Gadallah, M.A. Antitumor Exopolysaccharides Derived from Novel Marine Bacillus: Isolation, Characterization Aspect and Biological Activity. Asian Pacific J. Cancer Prev. 2017, 18, 1847–1854. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 922. [Google Scholar] [CrossRef]
- Amer, M.N.; Elgammal, E.W.; Atwa, N.A.; Eldiwany, A.I.; Dawoud, I.E.; Rashad, F.M. Structure Elucidation and in Vitro Biological Evaluation of Sulfated Exopolysaccharide from LAB Weissella paramesenteroides MN2C2. J. Appl. Pharm. Sci. 2021, 11, 22–31. [Google Scholar] [CrossRef]
- Sirin, S.; Aslim, B. Characterization of Lactic Acid Bacteria Derived Exopolysaccharides for Use as a Defined Neuroprotective Agent against Amyloid Beta1–42-Induced Apoptosis in SH-SY5Y Cells. Sci. Rep. 2020, 10, 8124. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, M.K.; Mishra, A.; Kumari, P.; Reddy, C.R.K.; Jha, B. Isolation and Characterization of Exopolysaccharides from Seaweed Associated Bacteria Bacillus licheniformis. Carbohydr. Polym. 2011, 84, 1019–1026. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and Anti-Biofilm Activity of Extracellular Polymeric Substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Jiang, G.; Gan, L.; Li, X.; He, J.; Zhang, S.; Chen, J.; Zhang, R.; Xu, Z.; Tian, Y. Characterization of Structural and Physicochemical Properties of an Exopolysaccharide Produced by Enterococcus Sp. F2 From Fermented Soya Beans. Front. Microbiol. 2021, 8, 922. [Google Scholar] [CrossRef]
- Boukhelata, N.; Taguett, F.; Kaci, Y. Characterization of an Extracellular Polysaccharide Produced by a Saharan Bacterium Paenibacillus tarimensis REG 0201M. Ann. Microbiol. 2019, 69, 93–106. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R. Phylogenetics and Antibacterial Properties of Exopolysaccharides from Marine Bacteria Isolated from Mauritius Seawater. Ann. Microbiol. 2019, 69, 957–972. [Google Scholar] [CrossRef]
- Spanò, A.; Laganà, P.; Visalli, G.; Maugeri, T.L.; Gugliandolo, C. In Vitro Antibiofilm Activity of an Exopolysaccharide from the Marine Thermophilic Bacillus licheniformis T14. Curr. Microbiol. 2016, 72, 518–528. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and Properties of Two Novel Exopolysaccharides Synthesized by a Thermophilic Bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Kulcinskaja, E.; Ron, E.Y.C.; Björnsdóttir, S.; Friðjónsson, Ó.H.; Hreggviðsson, G.Ó.; Karlsson, E.N. Evaluation of the Production of Exopolysaccharides by Two Strains of the Thermophilic Bacterium Rhodothermus marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar] [CrossRef]
- Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust. Fermentation 2021, 7, 33. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal Properties of an Exopolysaccharide Produced by a Marine Thermotolerant Bacillus licheniformis by ATR-FTIR Spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Jayamurthy, H.; Dhar, K.S.; Pandey, A. Studies on Structural and Physical Characteristics of a Novel Exopolysaccharide from Pseudozyma Sp. NII 08165. Int. J. Biol. Macromol. 2013, 59, 84–89. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Qiao, Y.; Wang, X.; Zhang, Q.; Zhao, W.; Huang, L. Purification, Characterization and Anticancer Activities of Exopolysaccharide Produced by Rhodococcus erythropolis HX-2. Int. J. Biol. Macromol. 2020, 145, 646–654. [Google Scholar] [CrossRef]
- Sran, K.S.; Sundharam, S.S.; Krishnamurthi, S.; Roy Choudhury, A. Production, Characterization and Bio-Emulsifying Activity of a Novel Thermostable Exopolysaccharide Produced by a Marine Strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249. [Google Scholar] [CrossRef]
- Karadayi, Y.I.; Aykutoglu, G.; Arslan, N.P.; Baltaci, M.O.; Adiguzel, A.; Taskin, M. Production of Water-Soluble Sulfated Exopolysaccharide with Anticancer Activity from Anoxybacillus gonensis YK25. J. Chem. Technol. Biotechnol. 2021, 96, 1258–1266. [Google Scholar] [CrossRef]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial Characterization and Antioxidant and Antiproliferative Activities of the Aqueous Extracellular Polysaccharides from the Thermophilic Microalgae Graesiella sp. BMC Complement. Altern. Med. 2016, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. A Bioactive Exopolysaccharide from Marine Bacteria Alteromonas Sp. PRIM-28 and Its Role in Cell Proliferation and Wound Healing in Vitro. Int. J. Biol. Macromol. 2019, 131, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Arun, A.B.; Rekha, P.D. Sulfated Exopolysaccharide Produced by Labrenzia Sp. PRIM-30, Characterization and Prospective Applications. Int. J. Biol. Macromol. 2014, 69, 290–295. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Zhai, Z.; Chen, A.; Zhou, H.; Zhang, D.; Du, X.; Liu, Q.; Wu, X.; Cheng, J.; Chen, L.; Hu, F.; et al. Structural Characterization and Functional Activity of an Exopolysaccharide Secreted by Rhodopseudomonas palustris GJ-22. Int. J. Biol. Macromol. 2021, 167, 160–168. [Google Scholar] [CrossRef]
- Sengupta, D.; Datta, S.; Biswas, D. Surfactant Exopolysaccharide of Ochrobactrum pseudintermedium C1 Has Antibacterial Potential: Its Bio-Medical Applications in Vitro. Microbiol. Res. 2020, 236, 126466. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Di Lorenzo, F.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; De Castro, C. Liquid-State NMR Spectroscopy for Complex Carbohydrate Structural Analysis: A Hitchhiker’s Guide. Carbohydr. Polym. 2022, 277, 118885. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, J.; Wu, J.; Li, H.; Wu, C.; Yin, Z.; Xu, J.; Zhu, L.; Gao, M.; Zhan, X. Structural Characterization and in Vitro Evaluation of the Prebiotic Potential of an Exopolysaccharide Produced by Bacillus thuringiensis during Fermentation. LWT 2022, 163, 113532. [Google Scholar] [CrossRef]
- Li, J.; Wu, G.; Qin, C.; Chen, W.; Chen, G.; Wen, L. Structure Characterization and Otoprotective Effects of a New Endophytic Exopolysaccharide from Saffron. Molecules 2019, 24, 749. [Google Scholar] [CrossRef]
- Naumenko, O.I.; Guo, X.; Senchenkova, S.N.; Geng, P.; Perepelov, A.V.; Shashkov, A.S.; Liu, B.; Knirel, Y.A. Structure and Gene Cluster of the O-Antigen of Escherichia coli O54. Carbohydr. Res. 2018, 462, 34–38. [Google Scholar] [CrossRef]
- Sigida, E.N.; Fedonenko, Y.P.; Shashkov, A.S.; Arbatsky, N.P.; Zdorovenko, E.L.; Konnova, S.A.; Ignatov, V.V.; Knirel, Y.A. Elucidation of a Masked Repeating Structure of the O-Specific Polysaccharide of the Halotolerant Soil Bacteria Azospirillum halopraeferens Au4. Beilstein J. Org. Chem. 2016, 12, 636–642. [Google Scholar] [CrossRef]
- Xu, X.; Ruan, D.; Jin, Y.; Shashkov, A.S.; Senchenkova, S.N.; Kilcoyne, M.; Savage, A.V.; Zhang, L. Chemical Structure of Aeromonas Gum--Extracellular Polysaccharide from Aeromonas Nichidenii 5797. Carbohydr. Res. 2004, 339, 1631–1636. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Casillo, A.; Gharsallah, H.; Jemil, N.; Lanzetta, R.; Attia, H.; Corsaro, M.M. Isolation and Structural Characterization of Levan Produced by Probiotic Bacillus tequilensis-GM from Tunisian Fermented Goat Milk. Int. J. Biol. Macromol. 2019, 133, 786–794. [Google Scholar] [CrossRef]
- Young, I.D.; Nepogodiev, S.A.; Black, I.M.; Le Gall, G.; Wittmann, A.; Latousakis, D.; Visnapuu, T.; Azadi, P.; Field, R.A.; Juge, N.; et al. Lipopolysaccharide Associated with β-2,6 Fructan Mediates TLR4-Dependent Immunomodulatory Activity in Vitro. Carbohydr. Polym. 2022, 277, 118606. [Google Scholar] [CrossRef]
- Dahech, I.; Fakhfakh, J.; Damak, M.; Belghith, H.; Mejdoub, H.; Belghith, K.S. Structural Determination and NMR Characterization of a Bacterial Exopolysaccharide. Int. J. Biol. Macromol. 2013, 59, 417–422. [Google Scholar] [CrossRef]
- London, J.A.; Taylor, S.L.; Barsukov, I.; Cartmell, A.; Yates, E.A. Exploration of Expanded Carbohydrate Chemical Space to Access Biological Activity using Microwave-Induced Acid Condensation of Simple Sugars. RSC Adv. 2022, 12, 11075–11083. [Google Scholar] [CrossRef]
- Tvaroska, I.; Taravel, F.R. Carbon-Proton Coupling Constants In The Conformational Analysis of Sugar Molecules. Adv. Carbohydr. Chem. Biochem. 1995, 51, 15–61. [Google Scholar] [CrossRef]
- Köseoğlu, V.K.; Heiss, C.; Azadi, P.; Topchiy, E.; Güvener, Z.T.; Lehmann, T.E.; Miller, K.W.; Gomelsky, M. Listeria monocytogenes Exopolysaccharide: Origin, Structure, Biosynthetic Machinery and c-Di-GMP-Dependent Regulation. Mol. Microbiol. 2015, 96, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Kaci, Y.; Heyraud, A.; Barakat, M.; Heulin, T. Isolation and Identification of an EPS-Producing Rhizobium Strain from Arid Soil (Algeria): Characterization of Its EPS and the Effect of Inoculation on Wheat Rhizosphere Soil Structure☆☆The GenBank/EMBL/DDBJ Accession Number for the 16S RRNA Gene Sequenc. Res. Microbiol. 2005, 156, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Balzaretti, S.; Taverniti, V.; Guglielmetti, S.; Fiore, W.; Minuzzo, M.; Ngo, H.N.; Ngere, J.B.; Sadiq, S.; Humphreys, P.N.; Laws, A.P.; et al. A Novel Rhamnose-Rich Hetero-Exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells. Appl. Environ. Microbiol. 2017, 83, e02702-16. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.; Petrovsky, N. Observation of the Keto Tautomer of D-Fructose in D(2)O Using (1)H NMR Spectroscopy. Carbohydr. Res. 2012, 347, 136–141. [Google Scholar] [CrossRef]
- Toukach, P. V Bacterial Carbohydrate Structure Database 3: Principles and Realization. J. Chem. Inf. Model. 2011, 51, 159–170. [Google Scholar] [CrossRef]
- Biswas, J.; Ganguly, J.; Paul, A.K. Partial Characterization of an Extracellular Polysaccharide Produced by the Moderately Halophilic Bacterium Halomonas xianhensis SUR308. Biofouling 2015, 31, 735–744. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohammed Breig, S.J.; Gómez, A.; Sánchez-Arévalo, I.; González-Faune, P.; Sarkar, S.; Bandopadhyay, R.; Vuree, S.; Cornejo, J.; Tapia, J.; et al. Optimization and Characterization of a Novel Exopolysaccharide from Bacillus haynesii CamB6 for Food Applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef]
- Seyfert, U.T.; Biehl, V.; Schenk, J. In Vitro Hemocompatibility Testing of Biomaterials According to the ISO 10993-4. Biomol. Eng. 2002, 19, 91–96. [Google Scholar] [CrossRef]
- Standard Practice for Assessment of Hemolytic Properties of Materials. Available online: https://www.astm.org/f0756-17.html (accessed on 9 September 2022).
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane Stabilization as a Mechanism of the Anti-Inflammatory Activity of Methanol Extract of Garden Egg (Solanum Aethiopicum). DARU J. Pharm. Sci. 2012, 20, 1. [Google Scholar] [CrossRef]
- Anosike, C.A.; Igboegwu, O.N.; Nwodo, O.F.C. Antioxidant Properties and Membrane Stabilization Effects of Methanol Extract of Mucuna pruriens Leaves on Normal and Sickle Erythrocytes. J. Tradit. Complement. Med. 2019, 9, 278–284. [Google Scholar] [CrossRef]
- Ali, P.; Shah, A.A.; Hasan, F.; Hertkorn, N.; Gonsior, M.; Sajjad, W.; Chen, F. A Glacier Bacterium Produces High Yield of Cryoprotective Exopolysaccharide. Front. Microbiol. 2020, 10, 3096. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Structural Elucidation and Bioactivity of a Novel Exopolysaccharide from Endophytic Fusarium solani SD5. Carbohydr. Polym. 2012, 90, 683–689. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Ashcraft, M.; Douglass, M.; Chen, Y.; Handa, H. Combination Strategies for Antithrombotic Biomaterials: An Emerging Trend towards Hemocompatibility. Biomater. Sci. 2021, 9, 2413–2423. [Google Scholar] [CrossRef]
- Sathishkumar, R.; Kannan, R.; Jinendiran, S.; Sivakumar, N.; Selvakumar, G.; Shyamkumar, R. Production and Characterization of Exopolysaccharide from the Sponge-Associated Bacillus subtilis MKU SERB2 and Its in-Vitro Biological Properties. Int. J. Biol. Macromol. 2021, 166, 1471–1479. [Google Scholar] [CrossRef]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, High Production and Antimicrobial Activity of Exopolysaccharides from Lactococcus lactis F-Mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Song, S.; Zhou, D.; Qiao, W.; Zhu, C.; Liu, S.; Zhu, B. Anticoagulant Activity and Structural Characterization of Polysaccharide from Abalone (Haliotis Discus Hannai Ino) Gonad. Molecules 2016, 21, 697. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q.; et al. Isolation, Purification, Characterization and Immunostimulatory Activity of an Exopolysaccharide Produced by Lactobacillus pentosus LZ-R-17 Isolated from Tibetan Kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef]










| S. No | Time (h) | Dry Cell Weight (g L−1) | EPS (g L−1) |
|---|---|---|---|
| 1 | 24 | 8.253 ± 0.533 | 9.327 ± 0.621 |
| 2 | 48 | 12.307 ± 0.603 | 13.333 ± 0.474 |
| 3 | 72 | 13.927 ± 0.783 | 15.680 ± 0.675 |
| 4 | 96 | 13.260 ± 0.623 | 17.333 ± 0.565 |
| S. No | Monosaccharides | VITP14 EPS (%) |
|---|---|---|
| 1 | Glucose | 25.8 |
| 2 | Ribose | 18.6 |
| 3 | Fructose | 31.5 |
| 4 | Xylose | 24.0 |
| Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | CH3 |
|---|---|---|---|---|---|---|---|
| A) → 2)-α-D-Glcp-(1 → | 5.15 96.07 (180 Hz) | 3.87 82.45 | 3.80 82.60 | 3.73 75.35 | 3.82 80.83 | 3.68 70.02 | - - |
| B) α-D-Xylp-(1 → | 5.02 100.86 (183 Hz) | 3.75 82.41 | 3.96 71.56 | 3.83 74.08 | 3.57 69.93 | - - | - - |
| C) → 2,4)-β-D-Ribp-(1 → | 4.96 92.22 (165 Hz) | 3.28 72.20 | 3.47 71.80 | 3.34 69.65 | 3.44 65.03 | - - | 1.03 17.19 |
| D) → 2,4,5)-β-D-Xylp-(1 → | 4.72/4.74 93.90 (163 Hz) | 3.30 72.31 | 3.85 70.10 | 3.65 70.64 | 3.49 67.65 | - - | - - |
| D′) β-D-Xylp-(1 → | 4.66 93.46 (161 Hz) | 3.56 70.31 | 3.72 70.11 | 3.66 69.81 | 3.62 67.48 | - - | - - |
| E) → 6)-β-D-Glcp-(1 → | 4.32/4.34 96.78 (159 Hz) | 3.00 74.13 | 3.11 76.01 | 3.20 75.92 | 3.37 69.56 | 3.70 67.92 | - - |
| F) → 1)-β-D-Frup-(2 → | 3.48/3.61 61.02 | - 98.04 | 3.79 68.52 | 3.85 69.20 | 3.88 75.15 | 3.51/3.58 61.50 | - - |
| G) → 6)-β-D-Fruf-(2 → | 3.71/3.59 62.40 | - 101.61 | 3.97 70.59 | 3.86 69.45 | 3.76 81.03 | 3.77/3.64 63.05 | - - |
| Residues | HMBC | Chemical Shifts | |
|---|---|---|---|
| From | To | Linkage | (δ, ppm) |
| A (H-1) A (C1) | E (C-6) E (H-6) | A (1 → 6) E α-D-Glcp-(1 → 6)-β-D-Glcp | AC1 (96.17)–EH6 (3.70) |
| B (H-1) B (C1) | A (C-2) A (H-2) | B (1 → 2) A α-D-Xylp-(1 → 2)-α-D-Glcp | BC1 (5.02)–AH2 (82.45) |
| C (H-1) C (C1) | F (C-1) F (H1) | C (1 → 1) F β-D-Ribp-(1 → 1)-β-D-Frup | CH1 (4.96)–FC1 (61.00) |
| D (H1) D (C1) | G (C-6) G (H-6) | D (1 → 6) G β-D-Xylp-(1 → 6)-β-D-Fruf | DH1 (4.72)–GC6 (63.10) |
| D′ (H1) D′ (C1) | D (C-4) D (H-4) | D′ (1 → 4) D β-D-Xylp-(1 → 4)-β-D-Xylp | D′C1 (93.60)–DH4 (3.65) |
| E (H1) E (C1) | F (C-5) F (H-5) | E (1 → 5) F β-D-Glcp-(1 → 5)-β-D-Frup | EH1 (4.34)–FC5 (75.13) |
| F (nd) F (C2) | D (C-2) D (H-2) | F (2 → 2) D β-D-Frup-(2 → 2)-β-D-Xylp | FC2 (98.01)–DH2 (3.30) |
| G (nd) G (C2) | C (C-4) C (H-4) | G (2 → 4) C β-D-Fruf-(2 → 4)-β-D-Ribp | GC2 (101.61)–CH4 (3.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrew, M.; Jayaraman, G. Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 2022, 14, 3986. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14193986
Andrew M, Jayaraman G. Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers. 2022; 14(19):3986. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14193986
Chicago/Turabian StyleAndrew, Monic, and Gurunathan Jayaraman. 2022. "Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast" Polymers 14, no. 19: 3986. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14193986