Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
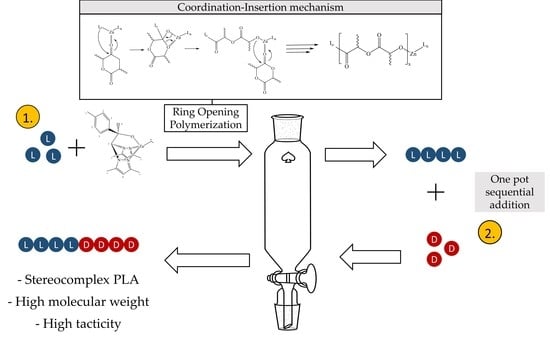
2.2. Synthesis
2.3. Instrumentation and Measurements
3. Results and Discussion
3.1. Characterization and Activity of the Initiator 1
3.2. Polymerization Mechanism
3.3. Polymerization Kinetics
3.4. Homochiral and Racemic Polymerization of Lactide
3.5. Stereo-Block Copolymerization by Sequential Monomer Addition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. ChemInform 2004, 36, 20–23. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 3–36. [Google Scholar]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Pan, P.; Han, L.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(L-lactic acid)/poly(D-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Kobayashi, K.; Kimura, Y. Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent D/L ratios. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 794–801. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Li, L.; Fan, Z.; Li, S. Stereocomplexed three-arm PPO–PDLA–PLLA copolymers: Synthesis via an end-functionalized initiator. Eur. Polym. J. 2014, 55, 27–34. [Google Scholar] [CrossRef]
- Masutani, K.; Lee, C.W.; Kimura, Y. Synthesis and thermomechanical properties of stereo triblock polylactides with nonequivalent block compositions. Macromol. Chem. Phys. 2012, 213, 695–704. [Google Scholar] [CrossRef]
- Masutani, K.; Lee, C.W.; Kimura, Y. Synthesis and properties of stereo di- and tri-block polylactides of different block compositions by terminal Diels-Alder coupling of poly-L-lactide and poly-D-lactide prepolymers. Polym. J. 2013, 45, 427–435. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. A novel synthetic approach to stereo-block poly(lactic acid). Macromol. Symp. 2005, 224, 133–144. [Google Scholar] [CrossRef]
- Fukushima, K.; Furuhashi, Y.; Sogo, K.; Miura, S.; Kimura, Y. Stereoblock poly(lactic acid): Synthesis via solid-state polycondensation of a stereocomplexed mixture of poly(L-lactic acid) and poly(D-lactic acid). Macromol. Biosci. 2005, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Polyesters from Dilactones. In Handbook of Ring-Opening Polymerization; Dubois, P., Coulembier, O., Raquez, J.-M., Eds.; WILEY-VCH Verlag GmbH & C: Weinheim, Germany, 2009; pp. 255–286. [Google Scholar]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Osado, A.G.; Sánchez-Barba, L.F.; Fernández-Baeza, J.; Otero, A.; Sánchez, A.L.; Rodríguez, A.M. Studies on Multinuclear Magnesium tert-Butyl Heteroscorpionates: Synthesis, Coordination Ability, and Heteroselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2017, 36, 884–897. [Google Scholar] [CrossRef]
- Sánchez-Barba, L.F.; Garcés, A.; Fernández-Baeza, J.; Otero, A.; Alonso-Moreno, C.; Lara-Sánchez, A.; Rodríguez, A.M. Stereoselective production of poly(rac-lactide) by ROP with highly efficient bulky heteroscorpionate alkylmagnesium initiators. Organometallics 2011, 30, 2775–2789. [Google Scholar] [CrossRef]
- Char, J.; Brulé, E.; Gros, P.; Rager, M.-N.; Guérineau, V.; Thomas, C.M. Synthesis of heterotactic PLA from rac-lactide using hetero-bimetallic Mg/Zn–Li systems. J. Organomet. Chem. 2015, 796, 47–52. [Google Scholar] [CrossRef]
- Yui, N.; Dijkstra, P.J.; Feijen, J. Stereo block copolymers of L- and D-lactides. Makromol. Chem. 1990, 191, 481–488. [Google Scholar] [CrossRef]
- Szwarc, M. Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Dyadkin, V.; Pattison, P.; Dmitriev, V.; Chernyshov, D. A new multipurpose diffractometer PILATUS@SNBL. J. Synchrotron Radiat. 2016, 23, 825–829. [Google Scholar] [CrossRef]
- Psciuk, B.T.; Lord, R.L.; Winter, C.H.; Schlegel, H.B. Can Metallapyrimidines Be Aromatic? A Computational Study into a New Class of Metallacycles. J. Chem. Theory Comput. 2012, 8, 4950–4959. [Google Scholar] [CrossRef] [PubMed]
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock polymers: Panacea or Dandora’s box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Tejeda, J.; Honrado, M.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M.; Chiral, N. N, O-scorpionate zinc alkyls as effective and stereoselective initiators for the living ROP of lactides. Organometallics 2012, 31, 4191–4202. [Google Scholar] [CrossRef]
- Laine, A.; Linnolahti, M.; Pakkanen, T.A.; Severn, J.R.; Kokko, E.; Pakkanen, A. Comparative Theoretical Study on Homopolymerization of α-Olefins by Bis(cyclopentadienyl) Zirconocene and Hafnocene: Elemental Propagation and Termination Reactions between Monomers and Metals. Organometallics 2010, 29, 1541–1550. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Sin, L.T.; Tueen, B.S. Synthesis and Production of Poly(Lactic Acid). In Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA; William Andrew: Norwich, NY, USA, 2019; pp. 53–95. [Google Scholar] [CrossRef]
- Zhang, X.; Macdonald, D.A.; Goosen, M.F.A.; Mcauley, K.B. Mechanism of Lactide Polymerization in the presence of Stannous Octoate: The effect of hydroxy and carboxylic acis substances. J. Polym. Chem. 1994, 32, 2965–2970. [Google Scholar] [CrossRef]
- Platel, R.H.; Hodgson, L.M.; Williams, C.K. Biocompatible Initiators for Lactide Polymerization. Polym. Rev. 2008, 48, 11–63. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.-L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Lara-Sánchez, A.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez, A.M. Catalytic behaviour in the ring-opening polymerisation of organoaluminiums supported by bulky heteroscorpionate ligands. Dalton Trans. 2015, 44, 12388–12400. [Google Scholar] [CrossRef]
- Huang, S.J.; Onyari, J.M. Multicomponent Polymers of Poly(Lactic Acid) Macromonomers with Methacrylate Terminal and Copolymers of Poly(2-Hydroxyethyl Methacrylate). J. Macromol. Sci. Pure Appl. Chem. 1996, 33, 571–584. [Google Scholar] [CrossRef]
- Sawai, D.; Tsugane, Y.; Tamada, M.; Kanamoto, T.; Sungil, M.; Hyon, S.-H. Crystal density and heat of fusion for a stereo-complex of poly(L-lactic acid) and poly(D-lactic acid). J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2632–2639. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; de Arenaza, I.M.; Meaurio, E.; Sarasua, J.R. Efficient stereocomplex crystallization in enantiomeric blends of high molecular weight polylactides. RSC Adv. 2015, 5, 34525–34534. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 4. Differential scanning calorimetric studies on precipitates from mixed solutions of poly(D-lactic acid) and poly(L-lactic acid). Macromolecules 1991, 24, 5657–5662. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Alterio, M.C.; Tedesco, C.; Pellecchia, C. Tailor-made block copolymers of l-, d- and rac-lactides and ε-caprolactone via one-pot sequential ring opening polymerization by pyridylamidozinc(ii) catalysts. RSC Adv. 2019, 9, 32771–32779. [Google Scholar] [CrossRef] [Green Version]
- Rosen, T.; Goldberg, I.; Navarra, W.; Venditto, V.; Kol, M. Divergent [{ONNN}Mg–Cl] complexes in highly active and living lactide polymerization. Chem. Sci. 2017, 8, 5476–5481. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-F.; Huang, Y.-F.; Ruan, J.; Su, A.-C. Extensive Development of Precursory Helical Pairs Prior to Formation of Stereocomplex Crystals in Racemic Polylactide Melt Mixture. Macromolecules 2012, 45, 872–878. [Google Scholar] [CrossRef]
- Tashiro, K.; Kouno, N.; Wang, H.; Tsuji, H. Crystal Structure of Poly(lactic acid) Stereocomplex: Random Packing Model of PDLA and PLLA Chains As Studied by X-ray Diffraction Analysis. Macromolecules 2017, 50, 8048–8065. [Google Scholar] [CrossRef]








| Entry | Sample | [LA]/[cat] | Temperature (°C) | Time (min) | Conv. (%) | Mn (theor.) (Da) a | Mn (exp.) (Da) b | PDI | (α) c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PLLA100 | 100 | 50 | 120 | 0 | 0 | - | - | |
| 2 | PLLA100 | 100 | 50 | 120 | 5 | 720 | - | - | |
| 3 | PLLA100 (L1) | 100 | 70 | 60 | 43 | 6192 | 12,824 | 1.36 | −159.4 |
| 4 | PLLA100 (L2) | 100 | 90 | 60 | 94 | 13,536 | 30,055 | 1.62 | −171.4 |
| 5 | PLLA500 (L3) | 500 | 90 | 45 | 94 | 67,680 | 62,564 | 1.84 | −172.2 |
| 6 | PLLA500 (L4) | 500 | 70 | 90 | 76 | 54,720 | 42,880 | 1.7 | −162.7 |
| 7 | PLLA500 (L5) | 500 | 70 | 120 | 94 | 67,680 | 48,253 | 2.19 | −168.4 |
| 8 | PDLA500 (D1) | 500 | 70 | 90 | 85 | 61,200 | 41,292 | 1.78 | 154 |
| 9 | rac-PLA500 (Rac1) | 500 | 70 | 120 | 90 | 64,800 | 14,212 | 1.66 | −1.1 |
| Entry | Sample | Time (min) a | Conv. (%) | Mn (theor.) (Da) b | Mn (exp.) (Da) c | Tm (°C) d | ΔHm (J/g) e | PDI | Pm f | (α) g |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (L50:D50) | 50 | 95 | 13,680 | 17,007 | 160–195.85 | 4.66–52.7 | 1.93 | 0.88 | 2.47 |
| 2 | (L100:D100) | 60 | 95 | 27,360 | 35,253 | 193.93 | 44.91 | 2.25 | 0.90 | 1.07 |
| 3 | (L300:D300) | 100 | 97 | 83,808 | 63,853 | 213.34 | 58.65 | 1.69 | 0.95 | 0.69 |
| 4 | (L500:D500) | 120 | 85 | 122,400 | 64,976 | 215.33 | 57.03 | 2.31 | 0.92 | 1.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Lopez, C.; Bravo, I.; Castro-Osma, J.A.; Chapron, D.; Bourson, P.; Vagner, C.; Cochez, M.; Leoné, N.; Lara-Sánchez, A.; Alonso-Moreno, C.; et al. Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst. Polymers 2022, 14, 232. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020232
Moya-Lopez C, Bravo I, Castro-Osma JA, Chapron D, Bourson P, Vagner C, Cochez M, Leoné N, Lara-Sánchez A, Alonso-Moreno C, et al. Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst. Polymers. 2022; 14(2):232. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020232
Chicago/Turabian StyleMoya-Lopez, Carmen, Ivan Bravo, José A. Castro-Osma, David Chapron, Patrice Bourson, Christelle Vagner, Marianne Cochez, Nils Leoné, Agustín Lara-Sánchez, Carlos Alonso-Moreno, and et al. 2022. "Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst" Polymers 14, no. 2: 232. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020232