Tailoring and Long-Term Preservation of the Properties of PLA Composites with “Green” Plasticizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Plasticized Composites
2.2.1. Melt Compounding with Torque Measuring Mixers
2.2.2. Reactive Melt Blending with Torque Measuring Mixers
2.2.3. Reactive Extrusion Using Twin-Screw Extruders (TSE)
2.2.4. Extrusion Laboratory Tests to Produce Tubes (Straws) and Films
2.3. Methods of Characterization
3. Results and Discussion
3.1. Effects of TBC Addition on the Properties of Composites
3.1.1. Rheology: Evolution of Torque during Melt Mixing
3.1.2. Morphology of Composites
3.1.3. Thermal Properties: DSC and TGA
3.1.4. Mechanical Properties
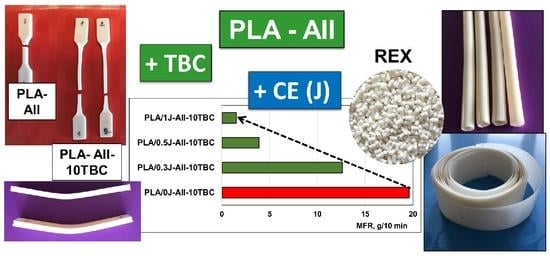
3.2. Tailoring the Melt Viscosity of Composites by Reactive Blending
3.3. Current Prospects: Plasticized Composites Produced by REX
3.3.1. Characterization of Composites Produced by REX
3.3.2. Characterization of Aged Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreekumar, K.; Bindhu, B.; Veluraja, K. Perspectives of polylactic acid from structure to applications. Polym. Renew. Resour. 2021, 12, 60–74. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards controlled degradation of poly(lactic) acid in technical applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Dubois, P. Reactive extrusion (rex): Using chemistry and engineering to solve the problem of ocean plastics. Engineering 2022, 14, 15–18. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Saedi Ardahaei, A.; Dai, Y.; Neisiany, R.E.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The life cycle assessment for polylactic acid (PLA) to make it a low-carbon material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Davies, S. Life cycle inventory and impact assessment data for 2014 Ingeo™ polylactide production. Ind. Biotechnol. 2015, 11, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Widiastuti, I. Polylactide nanocomposites for packaging materials: A review. AIP Conf. Proc. 2016, 1710, 030020. [Google Scholar] [CrossRef]
- Kirac, F.T.; Dagdelen, A.F.; Saricaoglu, F.T. Recent advances in polylactic acid biopolymer films used in food packaging systems. J. Food Nutr. Res. 2022, 61, 1–15. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Avinc, O.; Khoddami, A. Overview of Poly(lactic acid) (PLA) fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Van den Oever, M.; Molenveld, K. Replacing fossil based plastic performance products by bio-based plastic products—technical feasibility. New Biotechnol. 2017, 37, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable polylactic acid (PLA)-based sustainable engineered blends and biocomposites: Recent developments, challenges, and opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Present situation and future perspectives of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost competitiveness of sustainable bioplastic feedstocks—A Monte Carlo analysis for polylactic acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Nieddu, E.; Mazzucco, L.; Gentile, P.; Benko, T.; Balbo, V.; Mandrile, R.; Ciardelli, G. Preparation and biodegradation of clay composites of PLA. React. Funct. Polym. 2009, 69, 371–379. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, B.H.; Choi, J.H.; Yoon, J.-S. Mechanical properties and thermal stability of poly(l-lactide)/calcium carbonate composites. J. Appl. Polym. Sci. 2008, 109, 3087–3092. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.; Ferraris, E.; Zhang, J. Melt crystallization of PLA/talc in fused filament fabrication. Mater. Des. 2019, 182, 108013. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Selective location of kaolin and effects of maleic anhydride in kaolin/poly(ε-caprolactone)/poly(lactic acid) composites. Appl. Clay Sci. 2020, 189, 105524. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Laoutid, F.; Dubois, P. Recent advances in production of ecofriendly polylactide (PLA)-calcium sulfate (anhydrite II) composites: From the evidence of filler stability to the effects of PLA matrix and filling on key properties. Polymers 2022, 14, 2360. [Google Scholar] [CrossRef]
- YousefniaPasha, H.; Mohtasebi, S.S.; Tabatabaeekoloor, R.; Taherimehr, M.; Javadi, A.; Soltani Firouz, M. Preparation and characterization of the plasticized polylactic acid films produced by the solvent-casting method for food packaging applications. J. Food Process. Preserv. 2021, 45, e16089. [Google Scholar] [CrossRef]
- Zych, A.; Perotto, G.; Trojanowska, D.; Tedeschi, G.; Bertolacci, L.; Francini, N.; Athanassiou, A. Super tough polylactic acid plasticized with epoxidized soybean oil methyl ester for flexible food packaging. ACS Appl. Polym. Mater. 2021, 3, 5087–5095. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Pluta, M.; Bonnaud, L.; Alexandre, M.; Dubois, P. Polylactide (PLA)–CaSO4 composites toughened with low molecular weight and polymeric ester-like plasticizers and related performances. Eur. Polym. J. 2008, 44, 3842–3852. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLA-hydroxyapatite composites—Thermo-mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber toughening of polylactic acid (PLA) with poly(butylene adipate-co-terephthalate) (PBAT): Mechanical properties, fracture mechanics and analysis of ductile-to-brittle behavior while varying temperature and test speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(l-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, N.; Wesslén, B. Tributyl citrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing effects of citrate esters on properties of poly(lactic acid). J. Polym. Eng. 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Martino, V.P.; Jiménez, A.; Ruseckaite, R.A. Processing and characterization of poly(lactic acid) films plasticized with commercial adipates. J. Appl. Polym. Sci. 2009, 112, 2010–2018. [Google Scholar] [CrossRef]
- Ruellan, A.; Ducruet, V.; Domenek, S. Chapter 5 Plasticization of poly(lactide). In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 124–170. [Google Scholar]
- Kfoury, G.; Raquez, J.-M.; Hassouna, F.; Odent, J.; Toniazzo, V.; Ruch, D.; Dubois, P. Recent advances in high performance poly(lactide): From “green” plasticization to super-tough materials via (reactive) compounding. Front. Chem. 2013, 1, 32. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, S.; Fritz, H.G. Plasticizing polylactide—the effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Alexandre, M.; Dubois, P. Polylactide (PLA) designed with desired end-use properties: 1. PLA compositions with low molecular weight ester-like plasticizers and related performances. Polym. Adv. Technol. 2008, 19, 636–646. [Google Scholar] [CrossRef]
- Abbott, S. Chemical compatibility of poly(lactic acid): A practical framework using Hansen solubility parameters. In Poly(lactic acid); Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 83–95. [Google Scholar]
- Detyothin, S.; Kathuria, A.; Jaruwattanayon, W.; Selke, S.E.M.; Auras, R. Poly(lactic acid) blends. In Poly(lactic acid); Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 227–271. [Google Scholar]
- Athanasoulia, I.-G.; Tarantili, P.A. Preparation and characterization of polyethylene glycol/poly(l-lactic acid) blends. Pure Appl. Chem. 2017, 89, 141–152. [Google Scholar] [CrossRef]
- Avolio, R.; Castaldo, R.; Avella, M.; Cocca, M.; Gentile, G.; Fiori, S.; Errico, M.E. PLA-based plasticized nanocomposites: Effect of polymer/plasticizer/filler interactions on the time evolution of properties. Compos. Part B Eng. 2018, 152, 267–274. [Google Scholar] [CrossRef]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirai, M.A.; Müller, C.M.O.; Grossmann, M.V.E.; Yamashita, F. Adipate and citrate esters as plasticizers for poly(lactic acid)/thermoplastic starch sheets. J. Polym. Environ. 2015, 23, 54–61. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F. Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J. Therm. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(l-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Chaos, A.; Sangroniz, A.; Gonzalez, A.; Iriarte, M.; Sarasua, J.-R.; del Río, J.; Etxeberria, A. Tributyl citrate as an effective plasticizer for biodegradable polymers: Effect of plasticizer on free volume and transport and mechanical properties. Polym. Int. 2019, 68, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Dou, Q. Crystallization behavior, morphology, and mechanical properties of poly(lactic acid)/tributyl citrate/treated calcium carbonate composites. Polym. Compos. 2014, 35, 1570–1582. [Google Scholar] [CrossRef]
- Lemmouchi, Y.; Murariu, M.; Santos, A.M.D.; Amass, A.J.; Schacht, E.; Dubois, P. Plasticization of poly(lactide) with blends of tributyl citrate and low molecular weight poly(d,l-lactide)-b-poly(ethylene glycol) copolymers. Eur. Polym. J. 2009, 45, 2839–2848. [Google Scholar] [CrossRef]
- Yang, Z.; Bi, H.; Bi, Y.; Rodrigue, D.; Xu, M.; Feng, X. Comparison between polyethylene glycol and tributyl citrate to modify the properties of wood fiber/polylactic acid biocomposites. Polym. Compos. 2019, 40, 1384–1394. [Google Scholar] [CrossRef]
- Menčík, P.; Přikryl, R.; Stehnová, I.; Melčová, V.; Kontárová, S.; Figalla, S.; Alexy, P.; Bočkaj, J. Effect of selected commercial plasticizers on mechanical, thermal, and morphological properties of poly(3-hydroxybutyrate)/Poly(lactic acid)/plasticizer biodegradable blends for three-dimensional (3D) print. Materials 2018, 11, 1893. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Toniazzo, V.; Dubois, P.; Ruch, D. New development on plasticized poly(lactide): Chemical grafting of citrate on PLA by reactive extrusion. Eur. Polym. J. 2012, 48, 404–415. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable micro and nano additives for controlling the migration of a biobased plasticizer from PLA-based flexible films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA–ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 48, 132. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive compatibilization of PLA/PA11 blends and their application in additive manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef] [Green Version]
- Standau, T.; Nofar, M.; Dörr, D.; Ruckdäschel, H.; Altstädt, V. A review on multifunctional epoxy-based Joncryl® ADR chain extended thermoplastics. Polym. Rev. 2022, 62, 296–350. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, morphological and mechanical studies of sustainably sourced polymer blends based on poly(lactic acid) and polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Duchet, J.; Reignier, J.; Maazouz, A. Melt strengthening of poly (lactic acid) through reactive extrusion with epoxy-functionalized chains. Rheol. Acta 2011, 50, 613–629. [Google Scholar] [CrossRef]
- Villalobos, M.; Awojulu, A.; Greeley, T.; Turco, G.; Deeter, G. Oligomeric chain extenders for economic reprocessing and recycling of condensation plastics. Energy 2006, 31, 3227–3234. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.-T. Effect of plasticizer on the crystallization behavior of poly(lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. Improving the ductility and heat deflection temperature of injection molded Poly(lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Varol, N.; Delpouve, N.; Araujo, S.; Domenek, S.; Guinault, A.; Golovchak, R.; Ingram, A.; Delbreilh, L.; Dargent, E. Amorphous rigidification and cooperativity drop in semi−crystalline plasticized polylactide. Polymer 2020, 194, 122373. [Google Scholar] [CrossRef]
- Wang, R.; Wan, C.; Wang, S.; Zhang, Y. Morphology, mechanical properties, and durability of poly(lactic acid) plasticized with di(isononyl) cyclohexane-1,2-dicarboxylate. Polym. Eng. Sci. 2009, 49, 2414. [Google Scholar] [CrossRef]
- Safandowska, M.; Rozanski, A.; Galeski, A. Plasticization of polylactide after solidification: An effectiveness and utilization for correct interpretation of thermal properties. Polymers 2020, 12, 561. [Google Scholar] [CrossRef] [Green Version]
- Wypych, G. 10—Effect of plasticizers on properties of plasticized materials. In Handbook of Plasticizers, 3rd ed.; Wypych, G., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; pp. 209–332. [Google Scholar]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rigid filler toughening in PLA-calcium carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2019, 113, 78–88. [Google Scholar] [CrossRef]
- Spear, M.J.; Eder, A.; Carus, M. 10—Wood polymer composites. In Wood Composites; Ansell, M.P., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 195–249. [Google Scholar]
- Mousavi, Z.; Heuzey, M.-C.; Randall, J.; Carreau, P.J. Enhanced properties of polylactide/polyamide 11 blends by reactive compatibilization. Can. J. Chem. Eng. 2022, 100, 2475–2490. [Google Scholar] [CrossRef]
- Han, J.; Zhang, M.; Zhang, H.; Liu, H.; Xu, S. Effects of modified tributyl citrate as a novel environmentally friendly plasticizer on the mechanical property and migration stability of soft polyvinyl chloride. J. Vinyl Addit. Technol. 2022, 28, 751–761. [Google Scholar] [CrossRef]
- Jaszkiewicz, A.; Bledzki, A.K.; van der Meer, R.; Franciszczak, P.; Meljon, A. How does a chain-extended polylactide behave?: A comprehensive analysis of the material, structural and mechanical properties. Polym. Bull. 2014, 71, 1675–1690. [Google Scholar] [CrossRef]

















| Sample Code | Composition, wt.% | ||
|---|---|---|---|
| PLA | AII | TBC | |
| PLA-AII | 70 | 30 | - |
| PLA-AII-5TBC | 65 | 30 | 5 |
| PLA-AII-10TBC | 60 | 30 | 10 |
| PLA-AII-15TBC | 55 | 30 | 15 |
| PLA-AII-20TBC | 50 | 30 | 20 |
| Sample | PLA–AII | PLA–AII–10TBC | PLA–AII–15TBC | PLA–AII–20TBC |
|---|---|---|---|---|
| MFR, g/10 min (190 °C, 2.16 kg) | 6.9 | 19.6 | 53.2 | NA (High fluidity) |
| Sample | DSC Cooling Scan, 10 °C/min | Second DSC Heating Scan, 10 °C/min | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tc, °C | ΔHc, J/g | χc, % | Tg, °C | Tcc, °C | ΔHcc, J/g | Tm, °C | ΔHm, J/g | χf, % | |
| PLA–AII | 95 | 5.1 | 5.5 | 60 | 107 | 23.3 | 170 | 36.5 | 14.2 |
| PLA–AII–5TBC | 83 | 9.6 | 10.3 | 44 | 85; 147 | 14.9; 2.7 | 166 | 40.2 | 24.3 |
| PLA–AII–10TBC | 75 | 24.2 | 26.0 | NA | – | – | 163 | 41.3 | 44.4 |
| PLA–AII–15TBC | 79 | 29.3 | 31.5 | NA | – | – | 160 | 40.2 | 43.2 |
| PLA–AII–20TBC | 82 | 33.5 | 36.0 | NA | – | – | 158 | 43.3 | 46.6 |
| Sample | Tg, °C | Tm, °C | DC, % |
|---|---|---|---|
| PLA–AII | 57 | 169 | 3.4 |
| PLA–AII–5TBC | 45 | 165 | 11.7 |
| PLA–AII–10TBC | 30 | 162 | 21.9 |
| PLA–AII–15TBC | 17 | 159 | 29.9 |
| PLA–AII–20TBC | ~3 | 155 | 37.1 |
| Sample | Onset of Thermal Degradation (T5%), °C | Temp. at Max. Rate of Degradation, °C | Residual Product at 600 °C, wt.% |
|---|---|---|---|
| PLA–AII | 345 | 378 | 30.3 |
| PLA–AII–5TBC | 320 | 374 | 30.0 |
| PLA–AII–10TBC | 290 | 375 | 30.4 |
| PLA–AII–15TBC | 253 | 362 | 29.0 |
| PLA–AII–20TBC | 234 | 365 | 29.6 |
| Properties | PLA/0.3J–AII–10TBC (TSE) | PLA–AII (TSE) |
|---|---|---|
| Mechanical properties | ||
| Tensile strength at yield, MPa | 38 (±1) | 47 (±1) |
| Tensile strength at break, MPa | 21 (±1) | 42 (±2) |
| Young’s modulus, MPa | 2400 (±100) | 3400 (±100) |
| Nominal elongation at yield, % | 3.2 (±0.1) | 2.8 (±0.1) |
| Nominal elongation at break, % | 111 (±3) | 3.5 (±0.4) |
| Max. flexural strength, MPa | 23 (±1) | 83 (±1) |
| Flexural modulus, MPa | 1400 (±60) | 5000 (±130) |
| Max. deflection, mm | >15 | 3.2/with break |
| Izod impact resistance, kJ/m2 | 4.9 (±0.7) | 3.1 (±0.1) |
| Thermal properties | ||
| Glass transition temperature, °C | 33 | 62 |
| Peak of melting temperature, °C | 165 | 171 |
| Onset of thermal degradation (T5%), °C | 278 | 331 |
| Propriety | Initial Films | Aged Films (2 Years) |
|---|---|---|
| Tensile strength at break, MPa | 25 (±2) | 27 (±2) |
| Nominal strain at break, % | 200 (±12) | 130 (±15) |
| Young’s modulus, MPa | 2600 (±200) | 2400 (±150) |
| Glass transition temperature, °C | 31 | ~30 ** |
| Peak of melting temperature, °C | 162 | 163 |
| Degree of crystallinity, % | 19.7 | 26.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murariu, M.; Paint, Y.; Murariu, O.; Laoutid, F.; Dubois, P. Tailoring and Long-Term Preservation of the Properties of PLA Composites with “Green” Plasticizers. Polymers 2022, 14, 4836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14224836
Murariu M, Paint Y, Murariu O, Laoutid F, Dubois P. Tailoring and Long-Term Preservation of the Properties of PLA Composites with “Green” Plasticizers. Polymers. 2022; 14(22):4836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14224836
Chicago/Turabian StyleMurariu, Marius, Yoann Paint, Oltea Murariu, Fouad Laoutid, and Philippe Dubois. 2022. "Tailoring and Long-Term Preservation of the Properties of PLA Composites with “Green” Plasticizers" Polymers 14, no. 22: 4836. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14224836