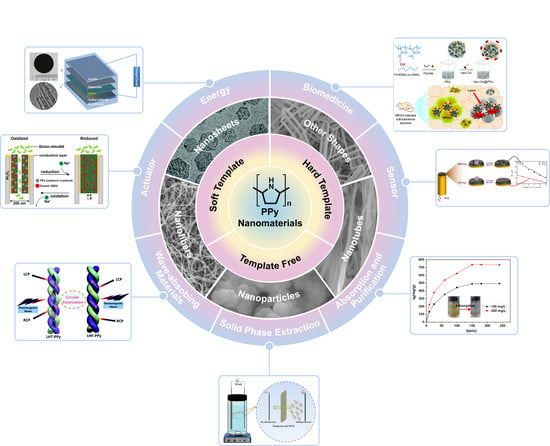
Polypyrrole Nanomaterials: Structure, Preparation and Application
Abstract
:1. Introduction
2. Types of PPy Nanomaterials
2.1. PPy Nanoparticles
2.2. PPy Nanotubes
2.3. PPy Nanowires
2.4. PPy Nanosheets
3. Preparation of PPy Nanomaterials
3.1. Soft Micellar Template Method
3.2. Hard Physical Template Method
3.3. Templateless Method
4. Application of PPy Nanomaterials
4.1. Energy Storage
4.1.1. Battery
4.1.2. Supercapacitor
| Morphology | Configuration | Capacitance | Cyclability | Ref. |
|---|---|---|---|---|
| Nanowire arrays | Symmetric capacitors | 699 F g−1 (1 A g−1) | 63% (5000 cycles, 50 A g−1) | [82] |
| Nanochains | Single electrode | 1502 F g−1 (2 mV s−1) | 93% (1500 cycles, 1 A g−1) | [100] |
| Nanowires | Single electrode | 328.7 F g−1 (0.3 A g−1) | 75.7% (600 cycles, 1.5 A g−1) | [107] |
| Nanofibers | Single electrode | 604 F g−1 (1.81 A g−1) | 91% (1000 cycles, 9 A g−1) | [115] |
| Hollow “horns” in micro/nanometers | Single electrode | 400 F g−1 (3 A g−1) | 90% (100,000 cycles, 500 mV s−1) | [117] |
| Nanobricks | Single electrode | 476 F g−1 (5 mV s−1) | [149] | |
| Nanoplates | Single electrode | 533 F g−1 (5 mV s−1) | 78% (5000 cycles, 100 mV s−1) | [150] |
| Nanosheets | Single electrode | 586 F g−1 (2 mV s−1) | 81% (5000 cycles, 100 mV s−1) | [152] |
| The clusters of nanofibers and nanoparticles | Single electrode | 427 F g−1 (0.02 A cm−1) | [153] | |
| Nanobrushes | Symmetric capacitors | 144.7 F g−1 (20 mV s−1) | 70% (20,000 cycles, 5 A g−1) | [155] |
| Nanowires | Single electrode | 420 F g−1 (1.5 A g−1) | 97.9 % (8000 cycles, 1.5 A g−1) | [156] |
| Films with hollow micro/nano-scaled horn arrays | Single electrode | 360 F g−1 (10 mV s−1) | 88.2% (10,000 cycles, 30 A g−1) | [157] |
| Nanospheres | Single electrode | 176 F g−1 (1 A g−1) | [158] | |
| Films with Micro/Nanosphere Shapes | Single electrode | 568 F g−1 (20 mV s−1) | 77% (10,000 cycles, 10 A g−1) | [159] |
| Hydrogels | Symmetric capacitors | 380 F g−1 (0.2 A g−1) | 90% (3000 cycles, 100 mV s−1) | [160] |
| 3D interconnected fibrous structure | Symmetric capacitors | 168 F g−1 (2 mA cm−2) | 97% (2000 cycles, 10 mV s−1) | [162] |
4.2. Biomedicine
4.2.1. Drug Delivery and Release
4.2.2. Photoacoustic and Photothermal Therapy
4.3. Sensors
4.3.1. Biosensors
- Introduced amino groups on an interdigitated microelectrode array (IDA) substrate;
- Immobilized carboxylated PPy nanomaterials on IDA substrate to maintain stable electrical contact between the PPy and the microelectrodes;
- Attached the aptamer to the surface of carboxylated PPy nanomaterials by coupling reaction.
- Acting as the grid dielectric of the p-type FET sensor, the target molecule specifically interacted with the adapter attached to the PPy surface.
4.3.2. Chemical Sensors
4.4. Others
4.4.1. Absorption and Impurity Removal
4.4.2. Wave-Absorbing Materials
4.4.3. Solid Phase Extraction
4.4.4. Actuators
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; Macdiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Jang, J. Conducting polymer nanomaterials and their applications. Adv. Polym. Sci. 2006, 199, 189–259. [Google Scholar]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, D.W.; Josowicz, M. Composites of intrinsically conducting polymers as sensing nanomaterials. Chem. Rev. 2008, 108, 746–769. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. Conducting-polymer nanomaterials for high-performance sensor applications: Issues and challenges. Adv. Funct. Mater. 2010, 19, 1567–1576. [Google Scholar] [CrossRef]
- Oh, W.K.; Kwon, O.S.; Jang, J. Conducting polymer nanomaterials for biomedical applications: Cellular interfacing and biosensing. Polym. Rev. 2013, 53, 407–442. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P. Water-dispersed polypyrrole nanospheres via chemical oxidative polymerization in the presence of castor oil sulfate. Synth. Met. 2010, 160, 345–350. [Google Scholar] [CrossRef]
- Woo, H.Y.; Jung, W.G.; Ihm, D.W.; Kim, J.Y. Synthesis and dispersion of polypyrrole nanoparticles in polyvinylpyrrolidone emulsion. Synth. Met. 2010, 160, 588–591. [Google Scholar] [CrossRef]
- Gupta, N.D.; Banerjee, D.; Das, N.S.; Chattopadhyay, K.K. Kinetics of micelle formation and their effect on the optical and structural properties of polypyrrole nanoparticles. Colloid Surface A 2011, 385, 55–62. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Wang, Y.; Liu, P. Polypyrrole nanoparticles with high dispersion stability via chemical oxidative polymerization in presence of an anionic–non-ionic bifunctional polymeric surfactant. Powder Technol. 2012, 217, 134–139. [Google Scholar] [CrossRef]
- Rawal, I.; Kaur, A. Effect of anionic surfactant concentration on the variable range hopping conduction in polypyrrole nanoparticles. J. Appl. Phys. 2014, 115, 043717. [Google Scholar] [CrossRef]
- Zhou, Z.; Shao, Y.; Gao, X.; Liu, Z.; Zhang, Q. Structural regulation of polypyrrole nanospheres guided by hydrophobic chain length of surfactants. J. Mater. Sci. 2019, 54, 14309–14319. [Google Scholar] [CrossRef]
- Grijalva-Bustamante, G.A.; Quevedo-Robles, R.V.; del Castillo-Castro, T.; Castillo-Ortega, M.M.; Encinas, J.C.; Rodriguez-Felix, D.E.; Lara-Ceniceros, T.E.; Fernandez-Quiroz, D.; Lizardi-Mendoza, J.; Armenta-Villegas, L. A novel bile salt -assisted synthesis of colloidal polypyrrole nanoparticles. Colloid Surface A 2020, 600, 124961. [Google Scholar] [CrossRef]
- Cruz, G.J.; Olayo, M.G.; Lopez, O.G.; Gomez, L.M.; Morales, J.; Olayo, R. Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer 2010, 51, 4314–4318. [Google Scholar] [CrossRef]
- Hazarika, J.; Kumar, A. Controllable synthesis and characterization of polypyrrole nanoparticles in sodium dodecylsulphate (SDS) micellar solutions. Synth. Met. 2013, 175, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yang, C.; Mu, B.; Liu, P. Effect of Acid Blue BRL on morphology and electrochemical properties of polypyrrole nanomaterials. Powder Technol. 2013, 235, 901–908. [Google Scholar] [CrossRef]
- Alizadeh, N.; Tavoli, F. Enhancing electrochromic contrast and redox stability of nanostructure polypyrrole film doped by heparin as polyanion in different solvents. J. Polym. Sci. Pol. Chem. 2014, 52, 3365–3371. [Google Scholar] [CrossRef]
- Jaworska, E.; Kisiel, A.; Michalska, A.; Maksymiuk, K. Electrochemical properties of polypyrrole nanoparticles -the role of doping ions and synthesis medium. Electroanalysis 2018, 30, 716–726. [Google Scholar] [CrossRef]
- Minisy, I.M.; Bober, P.; Sedenkova, I.; Stejskal, J. Methyl red dye in the tuning of polypyrrole conductivity. Polymer 2020, 207, 122854. [Google Scholar] [CrossRef]
- Liao, Y.; Li, X.; Kaner, R.B. Facile synthesis of water-dispersible conducting polymer nanospheres. Acs Nano 2010, 4, 5193–5202. [Google Scholar] [CrossRef]
- Ning, X.; Zhong, W.; Li, S.; Wan, L. A novel approach for the synthesis of monodispere polypyrrole nanospheres. Mater. Lett. 2014, 117, 294–297. [Google Scholar] [CrossRef]
- Pecher, J.; Mecking, S. Nanoparticles of conjugated polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Yoon, H.; Jang, J. Kinetic study of the formation of polypyrrole nanoparticles in water-soluble polymer/metal cation systems: A light-scattering analysis. Small 2010, 6, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Unstirred preparation of soluble electroconductive polypyrrole nanoparticles. Microchim. Acta 2010, 168, 205–213. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization model for hydrogen peroxide initiated synthesis of polypyrrole nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, K.; Lv, G.; Wang, G.; Yu, D.; Shao, J. UV-catalytic pPreparation of polypyrrole nanoparticles induced by H2O2. J. Phys. Chem. C 2015, 119, 18707–18718. [Google Scholar] [CrossRef]
- Hao, L.; Zhu, K.; Zhang, S.; Yu, D. The green preparation of poly N-vinylpyrrole nanoparticles. RSC Adv. 2016, 6, 90354–90359. [Google Scholar] [CrossRef]
- Hong, J.Y.; Jeon, S.O.; Jang, J.; Song, K.; Kim, S.H. A facile route for the preparation of organic bistable memory devices based on size-controlled conducting polypyrrole nanoparticles. Org. Electron. 2013, 14, 979–983. [Google Scholar] [CrossRef]
- Kim, S.; Oh, W.K.; Jeong, Y.S.; Hong, J.Y.; Cho, B.R.; Hahn, J.S.; Jang, J. Cytotoxicity of, and innate immune response to, size-controlled polypyrrole nanoparticles in mammalian cells. Biomaterials 2011, 32, 2342–2350. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, P.; Hu, M.; Tian, X. Charge carrier related superior capacitance of the precisely size-controlled polypyrrole nanoparticles. Electrochim. Acta 2017, 249, 290–300. [Google Scholar] [CrossRef]
- Kwon, O.S.; Hong, J.Y.; Park, S.J.; Jang, Y.; Jang, J. Resistive gas sensors based on precisely size-controlled polypyrrole nanoparticles: Effects of particle size and deposition method. J. Phys. Chem. C 2010, 114, 18874–18879. [Google Scholar] [CrossRef]
- Yang, M.; Wang, W.; Qiu, J.; Bai, M.; Xia, Y. Direct visualization and semi-quantitative analysis of payload loading in the case of gold nanocages. Angew. Chem. Int. Edit. 2019, 58, 17671–17674. [Google Scholar] [CrossRef]
- Lee, J.S.; Jun, J.; Shin, D.H.; Jang, J. Urchin-like polypyrrole nanoparticles for highly sensitive and selective chemiresistive sensor application. Nanoscale 2014, 6, 4188–4194. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Zhang, J.; Dou, S.; Wang, G. Polypyrrole hollow nanospheres: Stable cathode materials for sodium-ion batteries. Chem. Commun. 2015, 51, 16092–16095. [Google Scholar] [CrossRef]
- Bai, M.Y.; Xia, Y. Facile synthesis of double-shelled polypyrrole hollow particles with a structure similar to that of a thermal bottle. Macromol. Rapid Comm. 2010, 31, 1863–1868. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, L.; Wu, M.; Guo, Y.; Meng, S. A novel chemical synthesis of bowl-shaped polypyrrole particles. Mater. Lett. 2014, 126, 185–188. [Google Scholar] [CrossRef]
- Martin, C.R.; Van Dyke, L.S.; Cai, Z.; Liang, W. Template synthesis of organic microtubules. J. Am. Chem. Soc. 1990, 112, 8976–8977. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, Z.; Dai, T.; Lu, Y. Facile fabrication of functional polypyrrole nanotubes via a reactive self-degraded template. Macromol. Rapid Comm. 2005, 26, 1736–1740. [Google Scholar] [CrossRef]
- Long, Y.; Li, M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G.; Mentus, S.; Pasti, I.; Gavrilov, N.; Krstic, J.; Travas-Sejdic, J.; Strover, L.T.; Kopecka, J.; Moravkova, Z.; Trchova, M.; et al. Synthesis, characterization, and electrochemistry of nanotubular polypyrrole and polypyrrole-derived carbon nanotubes. J. Phys. Chem. C 2014, 118, 14770–14784. [Google Scholar] [CrossRef]
- Varga, M.; Kopecka, J.; Moravkova, Z.; Krivka, I.; Trchova, M.; Stejskal, J.; Prokes, J. Effect of oxidant on electronic transport in polypyrrole nanotubes synthesized in the presence of methyl orange. J. Polym. Sci. Pol Phys. 2015, 53, 1147–1159. [Google Scholar] [CrossRef]
- Bober, P.; Stejskal, J.; Sedenkova, I.; Trchova, M.; Martinkova, L.; Marek, J. The deposition of globular polypyrrole and polypyrrole nanotubes on cotton textile. Appl. Surf. Sci. 2015, 356, 737–741. [Google Scholar] [CrossRef]
- Ying, S.; Zheng, W.; Li, B.; She, X.; Huang, H.; Li, L.; Huang, Z.; Huang, Y.; Liu, Z.; Yu, X. Facile fabrication of elastic conducting polypyrrole nanotube aerogels. Synth. Met. 2016, 218, 50–55. [Google Scholar] [CrossRef]
- Li, Y.; Bober, P.; Apaydin, D.H.; Syrovy, T.; Sariciftci, N.S.; Hromadkova, J.; Sapurina, I.; Trchova, M.; Stejskal, J. Colloids of polypyrrole nanotubes/nanorods: A promising conducting ink. Synth. Met. 2016, 221, 67–74. [Google Scholar] [CrossRef]
- Varga, M.; Kopecky, D.; Kopecka, J.; Krivka, I.; Hanus, J.; Zhigunov, A.; Trchova, M.; Vrnata, M.; Prokes, J. The ageing of polypyrrole nanotubes synthesized with methyl orange. Eur. Polym. J. 2017, 96, 176–189. [Google Scholar] [CrossRef]
- Sapurina, I.; Li, Y.; Alekseeva, E.; Bober, P.; Trchova, M.; Moravkova, Z.; Stejskal, J. Polypyrrole nanotubes: The tuning of morphology and conductivity. Polymer 2017, 113, 247–258. [Google Scholar] [CrossRef]
- Li, Y.; Bober, P.; Trchova, M.; Stejskal, J. Polypyrrole prepared in the presence of methyl orange and ethyl orange: Nanotubes versus globules in conductivity enhancement. J. Mater. Chem. C 2017, 5, 4236–4245. [Google Scholar] [CrossRef]
- Kopecky, D.; Varga, M.; Prokes, J.; Vrnata, M.; Trchova, M.; Kopecka, J.; Vaclavik, M. Optimization routes for high electrical conductivity of polypyrrole nanotubes prepared in presence of methyl orange. Synth. Met. 2017, 230, 89–96. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Lima, R.V.; Wolfart, F.; Vidotti, M. Influence of the pH on the electrochemical synthesis of polypyrrole nanotubes and the supercapacitive performance evaluation. Electrochim. Acta 2019, 293, 447–457. [Google Scholar] [CrossRef]
- Prokes, J.; Varga, M.; Vrnata, M.; Valtera, S.; Stejskal, J.; Kopecky, D. Nanotubular polypyrrole: Reversibility of protonation/deprotonation cycles and long-term stability. Eur. Polym. J. 2019, 115, 290–297. [Google Scholar] [CrossRef]
- Hryniewiez, B.M.; Lima, R.V.; Marchesi, L.F.; Vidotti, M. Impedimetric studies about the degradation of polypyrrole nanotubes during galvanostatic charge and discharge cycles. J. Electroanal. Chem. 2019, 885, 113636. [Google Scholar] [CrossRef]
- Mao, J.; Li, C.; Park, H.J.; Rouabhia, M.; Zhang, Z. Conductive polymer waving in liquid nitrogen. ACS Nano 2017, 11, 10409–10416. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A. Structural, thermal and dielectric studies of polypyrrole nanotubes synthesized by reactive self degrade template method. Mat. Sci. Eng. B Adv. 2013, 178, 982–989. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A. Engineering polypyrrole nanotubes by 100 MeV Si9+ ion beam irradiation: Enhancement of antioxidant activity. Mat. Sci. Eng. C Mater. 2013, 33, 4900–4904. [Google Scholar] [CrossRef]
- Upadhyay, J.; Gogoi, B.; Kumar, A.; Buragohain, A.K. Diameter dependent antioxidant property of polypyrrole nanotubes for biomedical applications. Mater. Lett. 2013, 102, 33–35. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A.; Gogoi, B.; Buragohain, A.K. Biocompatibility and antioxidant activity of polypyrrole nanotubes. Synth. Met. 2014, 189, 119–125. [Google Scholar] [CrossRef]
- Wei, M.; Dai, T.; Lu, Y. Controlled fabrication of nanostructured polypyrrole on ion association template: Tubes, rods and networks. Synth. Met. 2010, 160, 849–854. [Google Scholar] [CrossRef]
- Lee, K.J.; Min, S.H.; Oh, H.; Jang, J. Fabrication of polymer nanotubes containing nanoparticles and inside functionalization. Chem. Commun. 2011, 47, 9447–9449. [Google Scholar] [CrossRef]
- Hazarika, J.; Kumar, A. Electric modulus based relaxation dynamics and ac conductivity scaling of polypyrrole nanotubes. Synth. Met. 2014, 198, 239–247. [Google Scholar] [CrossRef]
- Xue, Y.; Lu, X.; Xu, Y.; Bian, X.; Kong, L.; Wang, C. Controlled fabrication of polypyrrole capsules and nanotubes in the presence of Rhodamine B. Polym. Chem. 2010, 1, 1602–1605. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Xia, C.; Wang, W.; Tian, F.; Zhou, Y. Preparation of a flexible polypyrrole nanoarray and its capacitive performance. Mater. Lett. 2014, 132, 417–420. [Google Scholar] [CrossRef]
- Wei, Y.; Mo, X.; Zhang, P.; Li, Y.; Liao, J.; Li, Y.; Zhang, J.; Ning, C.; Wang, S.; Deng, X.; et al. Directing stem cell differentiation via electrochemical reversible switching between nanotubes and nanotips of polypyrrole array. ACS Nano 2017, 11, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Trchova, M.; Stejskal, J. Resonance raman spectroscopy of conducting polypyrrole nanotubes: Disordered surface versus ordered body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- Minisy, I.M.; Bober, P.; Acharya, U.; Trchova, M.; Hromadkova, M.; Pfleger, J.; Stejskal, J. Cationic dyes as morphology-guiding agents for one-dimensional polypyrrole with improved conductivity. Polymer 2019, 174, 11–17. [Google Scholar] [CrossRef]
- Ge, D.; Mu, J.; Huang, S.; Gcilitshana, O.U.; Ji, S.; Linkov, V.; Shi, W. Electrochemical synthesis of polypyrrole nanowires in the presence of gelatin. Synth. Met. 2011, 161, 166–172. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, M.J.; Lim, S.J.; Song, I.S.; Kwon, H.S. Single-step synthesis of polypyrrole nanowires by cathodic electropolymerization. J. Mater. Chem. A 2013, 1, 8061–8068. [Google Scholar] [CrossRef]
- Lee, D.; Zhang, C.; Gao, H. Facile Production of Polypyrrole Nanofibers Using a Freeze-Drying Method. Macromol. Chem. Phys. 2014, 215, 669–674. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, W.; Liao, J.; Huang, S.; Chen, J.; He, T.; Tan, G.; Ning, C. Chondroitin sulphate-guided construction of polypyrrole nanoarchitectures. Mat. Sci. Eng. C Mater. 2015, 48, 172–178. [Google Scholar] [CrossRef]
- Ramirez, A.M.R.; Gacitua, M.A.; Ortega, E.; Diaz, F.R.; del Valle, M.A. Electrochemical in situ synthesis of polypyrrole nanowires. Electrochem. Commun. 2019, 102, 94–98. [Google Scholar] [CrossRef]
- Sun, X.; Lin, H.; Zhang, C.; Huang, X.; Jin, J.; Di, S. Electrosynthesized nanostructured polypyrrole on selective laser melted titanium scaffold. Surf. Coat. Technol. 2019, 370, 11–17. [Google Scholar] [CrossRef]
- Xia, X.; Yin, J.; Qiang, P.; Zhao, X. Electrorheological properties of thermo-oxidative polypyrrole nanofibers. Polymer 2011, 52, 786–792. [Google Scholar] [CrossRef]
- Ru, X.; Shi, W.; Huang, X.; Cui, X.; Ren, B.; Ge, D. Synthesis of polypyrrole nanowire network with high adenosine triphosphate release efficiency. Electrochim. Acta 2011, 56, 9887–9892. [Google Scholar] [CrossRef]
- Burgoyne, H.A.; Kim, P.; Kolle, M.; Epstein, A.K.; Aizenberg, J. Screening conditions for rationally engineered electrodeposition of nanostructures (SCREEN): Electrodeposition and applications of polypyrrole nanofibers using microfluidic gradients. Small 2012, 8, 3502–3509. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Mu, J.; Dai, L.; Zhang, Z.; Sun, Y.; Shi, W.; Ge, D. Synthesis of polypyrrole nanowires with positive effect on MC3T3-E1 cell functions through electrical stimulation. Mat. Sci. Eng. C Mater. 2017, 71, 43–50. [Google Scholar] [CrossRef]
- Nie, X.; Ji, B.; Chen, N.; Liang, Y.; Han, Q.; Qu, L. Gradient doped polymer nanowire for moistelectric nanogenerator. Nano Energy 2018, 46, 297–304. [Google Scholar] [CrossRef]
- Sun, M.; Xu, C.; Li, J.; Xing, L.; Zhou, T.; Wu, F.; Shang, Y.; Xie, A. Protonic doping brings tuneable dielectric and electromagnetic attenuated properties for polypyrrole nanofibers. Chem. Eng. J. 2020, 381, 122615. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymer nanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- Kim, J.T.; Seol, S.K.; Pyo, J.; Lee, J.S.; Je, J.H.; Margaritondo, G. Three-dimensional writing of conducting polymer nanowire arrays by meniscus-guided polymerization. Adv. Mater. 2011, 23, 1968–1970. [Google Scholar] [CrossRef]
- Xia, L.; Quan, B.; Wei, Z. Patterned growth of vertically aligned polypyrrole nanowire arrays. Macromol. Rapid Comm. 2011, 32, 1998–2002. [Google Scholar] [CrossRef]
- Atobe, M.; Yoshida, N.; Sakamoto, K.; Sugino, K.; Fuchigami, T. Preparation of highly aligned arrays of conducting polymer nanowires using templated electropolymerization in supercritical fluids. Electrochim. Acta 2013, 87, 409–415. [Google Scholar] [CrossRef]
- Sulka, G.D.; Hnida, K.; Brzozka, A. PH sensors based on polypyrrole nanowire arrays. Electrochim. Acta 2013, 104, 536–541. [Google Scholar] [CrossRef]
- Huang, Z.; Song, Y.; Xu, X.; Liu, X. Ordered polypyrrole nanowire arrays grown on a carbon cloth substrate for a high-performance pseudocapacitor electrode. ACS Appl. Mate. Inter. 2015, 7, 25506–25513. [Google Scholar] [CrossRef] [PubMed]
- Debiemme-Chouvy, C.; Fakhry, A.; Pillier, F. Electrosynthesis of polypyrrole nano/micro structures using an electrogenerated oriented polypyrrole nanowire array as framework. Electrochim. Acta 2018, 268, 66–72. [Google Scholar] [CrossRef]
- Xing, J.; Qi, S.; Wang, Z.; Yi, X.; Zhou, Z.; Chen, J.; Huang, S.; Tan, G.; Chen, D.; Yu, P.; et al. Antimicrobial peptide functionalized conductive nanowire array electrode as a promising candidate for bacterial environment application. Adv. Funct. Mater. 2019, 29, 1806353. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, Y.; Peng, H.; Li, L.J.; Wu, T.; Ma, J.; Boey, F.Y.C.; Chen, X.; Chi, L. Enhanced electrical conductivity of individual conducting polymer nanobelts. Small 2011, 7, 1949–1953. [Google Scholar] [CrossRef]
- Hentschel, C.; Jiang, L.; Ebeling, D.; Zhang, J.; Chen, X.; Chi, L. Conductance measurements of individual polypyrrole nanobelts. Nanoscale 2015, 7, 2301–2305. [Google Scholar] [CrossRef] [Green Version]
- Chartuprayoon, N.; Hangarter, C.M.; Rheem, Y.; Jung, H.; Myung, N.V. Wafer-scale fabrication of single polypyrrole nanoribbon-based ammonia sensor. J. Phys. Chem. C 2010, 114, 11103–11108. [Google Scholar] [CrossRef]
- Wen, P.; Tan, C.; Zhang, J.; Meng, F.; Jiang, L.; Sun, Y.; Chen, X. Chemically tunable photoresponse of ultrathin polypyrrole. Nanoscale 2017, 9, 7760–7764. [Google Scholar] [CrossRef]
- Jeon, S.S.; An, H.H.; Yoon, C.S.; Im, S.S. Synthesis of ultra-thin polypyrrole nanosheets for chemical sensor applications. Polymer 2011, 52, 652–657. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Liu, P. Well-defined polypyrrole nanoflakes via chemical oxidative polymerization in the presence of sodium alkane sulfonate. Mater. Lett. 2011, 65, 1448–1450. [Google Scholar] [CrossRef]
- Jha, P.; Koiry, S.P.; Saxena, V.; Veerender, P.; Chauhan, A.K.; Aswal, D.K.; Gupta, S.K. Growth of free-standing polypyrrole nanosheets at air/liquid interface using J-aggregate of porphyrin derivative as in-situ template. Macromolecules 2011, 44, 4583–4585. [Google Scholar] [CrossRef]
- Jha, P.; Ramgir, N.S.; Sharma, P.K.; Datta, N.; Kailasaganapathi, S.; Kaur, M.; Koiry, S.P.; Saxena, V.; Chauhan, A.K.; Debnath, A.K.; et al. Charge transport and ammonia sensing properties of flexible polypyrrole nanosheets grown at air–liquid interface. Mater. Chem. Phys. 2013, 140, 300–306. [Google Scholar] [CrossRef]
- Liu, S.; Gordiichuk, P.; Wu, Z.S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Huang, X. Tuning the morphologies and electrical properties of azobenzene-4,4’-dicarboxylate-doped polypyrrole via ultraviolet light irradiation and medium pH alteration. Polymer 2019, 176, 188–195. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Poyraz, S.; Surwade, S.P.; Manohar, S.K. Oxidative template for conducting polymer nanoclips. J. Am. Chem. Soc. 2010, 132, 13158–13159. [Google Scholar] [CrossRef]
- Oaki, Y.; Kijima, M.; Imai, H. Synthesis and morphogenesis of organic polymer materials with hierarchical structures in biominerals. J. Am. Chem. Soc. 2011, 133, 8594–8599. [Google Scholar] [CrossRef]
- Yang, M.H.; Hong, S.B.; Yoon, J.H.; Kim, D.S.; Jeong, S.W.; Yoo, D.E.; Lee, T.J.; Lee, K.G.; Lee, S.J.; Choi, B.G. Fabrication of flexible, redoxable, and conductive nanopillar arrays with enhanced electrochemical performance. ACS Appl. Mater. Inter. 2016, 8, 22220–22226. [Google Scholar] [CrossRef]
- Bao, B.; Hao, J.; Bian, X.; Zhu, X.; Xiao, K.; Liao, J.; Zhou, J.; Zhou, Y.; Jiang, L. 3D porous hydrogel/conducting polymer heterogeneous membranes with electro-/pH-modulated ionic rectification. Adv. Mater. 2017, 29, 1702926. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, L.; Chen, D.; Wang, Z.; Zhai, J.; Wang, R.; Tan, G.; Mao, J.; Yu, P.; Ning, C. Construction of high surface potential polypyrrole nanorods with enhanced antibacterial properties. J. Mater. Chem. B 2018, 6, 3128–3135. [Google Scholar] [CrossRef]
- Shehnaz; Wu, D.; Guo, Y.; Song, X.; Yang, Y.; Mao, Q.; Ren, S.; Hao, C. Synergistic effect of heat treatments and KOH activation enhances the electrochemistry performance of polypyrrole nanochains (PPy-NCs). Electrochim. Acta 2018, 266, 151–160. [Google Scholar] [CrossRef]
- Gan, D.; Han, L.; Wang, M.; Xing, W.; Xu, T.; Zhang, H.; Wang, K.; Fang, L.; Lu, X. Conductive and tough hydrogels based on biopolymer molecular templates for controlling in situ formation of polypyrrole nanorods. ACS Appl. Mater. Inter. 2018, 10, 36218–36228. [Google Scholar] [CrossRef] [PubMed]
- Santino, L.M.; Diao, Y.; Yang, H.; Lu, Y.; Wang, H.; Hwang, E.; D’Arcy, J.M. Vapor/liquid polymerization of ultraporous transparent and capacitive polypyrrole nanonets. Nanoscale 2019, 11, 12358–12369. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Qiu, J. Ordered polypyrrole nanorings with near-infrared spectrum absorption and photothermal conversion performance. Chem. Eng. J. 2019, 359, 652–661. [Google Scholar] [CrossRef]
- Han, Y.; Qing, X.; Ye, S.; Lu, Y. Conducting polypyrrole with nanoscale hierarchical structure. Synth. Met. 2010, 160, 1159–1166. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Sundaresan, V.B. Phospholipid vesicles as soft templates for electropolymerization of nanostructured polypyrrole membranes with long range order. J. Mater. Chem. A 2014, 2, 11784–11791. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Jiang, H.; Zhang, K.J.; Chang, K.; Jia, S.; Jiang, W.; Shang, Y.; Lu, W.; Deng, S.; et al. Polypyrrole nanoparticles fabricated via Triton X-100 micelles template approach and their acetone gas sensing property. Appl. Surf. Sci. 2013, 280, 212–218. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.; Zhang, S.; Dong, F.; Liu, H. Soft template interfacial growth of novel ultralong polypyrrole nanowires for electrochemical energy storage. Electrochim. Acta 2014, 132, 112–117. [Google Scholar] [CrossRef]
- Qu, Q.; Zhu, Y.; Gao, X.; Wu, Y. Core-shell structure of polypyrrole grown on V2O5 nanoribbon as high performance anode material for supercapacitors. Adv. Energy Mater. 2012, 2, 950–955. [Google Scholar] [CrossRef]
- Zhang, X.; Manohar, S.K. Narrow pore-diameter polypyrrole nanotubes. J. Am. Chem. Soc. 2005, 127, 14156–14157. [Google Scholar] [CrossRef]
- Zhan, Y.; He, S.; Wan, X.; Zhang, J.; Liu, B.; Wang, J.; Li, Z. Easy-handling bamboo-like polypyrrole nanofibrous mats with high adsorption capacity for hexavalent chromium removal. J. Colloid Interf. Sci. 2018, 529, 385–395. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Poyraz, S.; Zhang, X. Green-nano approach to nanostructured polypyrrole. Chem. Commun. 2011, 47, 4421–4423. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, J.; Li, Q.; Wang, X. Capsular polypyrrole hollow nanofibers: An efficient recyclable adsorbent for hexavalent chromium removal. J. Mater. Chem. A 2015, 3, 15124–15132. [Google Scholar] [CrossRef]
- Kowalski, D.; Schmuki, P. Polypyrrole self-organized nanopore arrays formed by controlled electropolymerization in TiO2 nanotube template. Chem. Commun. 2010, 46, 8585–8587. [Google Scholar] [CrossRef]
- Kowalski, D.; Tighineanu, A.; Schmuki, P. Polymer nanowires or nanopores? Site selective filling of titania nanotubes with polypyrrole. J. Mater. Chem. 2011, 21, 17909–17915. [Google Scholar] [CrossRef]
- Dubal, D.P.; Caban-Huertas, Z.; Holze, R.; Gomez-Romero, P. Growth of polypyrrole nanostructures through reactive templates for energy storage applications. Electrochim. Acta 2016, 191, 346–354. [Google Scholar] [CrossRef]
- Velazquez, J.M.; Gaikwad, A.V.; Rout, T.K.; Rzayev, J.; Banerjee, S. A Substrate-Integrated and Scalable Templated Approach Based on Rusted Steel for the Fabrication of Polypyrrole Nanotube Arrays. ACS Appl. Mater. Inter. 2011, 3, 1238–1244. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Yan, F.; Zhu, J.; Wang, J. Template-free prepared micro/nanostructured polypyrrole with ultrafast charging/discharging rate and long cycle life. J. Power Sources 2011, 196, 2373–2379. [Google Scholar] [CrossRef]
- Liao, J.; Wu, S.; Yin, Z.; Huang, S.; Ning, C.; Tan, G.; Chu, P.K. Surface-dependent self-assembly of conducting polypyrrole nanotube arrays in template-free electrochemical polymerization. ACS Appl. Mater. Inter. 2014, 6, 10946. [Google Scholar] [CrossRef]
- Fakhry, A.; Pillier, F.; Debiemme-Chouvy, C. Templateless electrogeneration of polypyrrole nanostructures: Impact of the anionic composition and pH of the monomer solution. J. Mater. Chem. A 2014, 2, 9859–9865. [Google Scholar] [CrossRef] [Green Version]
- Chebil, S.; Monod, M.O.; Fisicaro, P. Direct electrochemical synthesis and characterization of polypyrrole nano- and micro-snails. Electrochim. Acta 2014, 123, 527–537. [Google Scholar] [CrossRef]
- Fakhry, A.; Cachet, H.; Debiemme-Chouvy, C. Mechanism of formation of templateless electrogenerated polypyrrole nanostructures. Electrochim. Acta 2015, 179, 297–303. [Google Scholar] [CrossRef]
- Mccarthy, C.P.; Mcguinness, N.B.; Alcock-Earley, B.E.; Breslin, C.B.; Rooney, A.D. Facile template-free electrochemical preparation of poly[N-(2-cyanoethyl) pyrrole] nanowires. Electrochem. Commun. 2012, 20, 79–82. [Google Scholar] [CrossRef]
- Rickard, J.J.S.; Farrer, I.; Oppenheimer, P.G. Tunable nanopatterning of conductive polymers via electrohydrodynamic lithography. ACS Nano 2016, 10, 3865–3870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Hoeppener, S.; Schubert, U.S. Reversible nanopatterning on polypyrrole films by atomic force microscope electrochemical lithography. Adv. Funct. Mater. 2016, 26, 614–619. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, D.; He, F.; Ni, C.; Chi, L. Fabricating sub-100 nm conducting polymer nanowires by edge nanoimprint lithography. J. Colloid Interf. Sci. 2015, 458, 300–304. [Google Scholar] [CrossRef]
- Karim, M.R.; Lee, C.J.; Lee, M.S. Synthesis of conducting polypyrrole by radiolysis polymerization method. Polym. Advan. Technol. 2010, 18, 916–920. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Dazzi, A.; Lefrancois, P.; Gervais, M.; Neron, S.; Remita, S. Radiolytic method as a novel approach for the synthesis of nanostructured conducting polypyrrole. Langmuir 2014, 30, 14086–14094. [Google Scholar] [CrossRef]
- Palaniappan, S.; Manisankar, P. Rapid synthesis of polypyrrole nanospheres by greener mechanochemical route. Mater. Chem. Phys. 2010, 122, 15–17. [Google Scholar] [CrossRef]
- Vetter, C.A.; Suryawanshi, A.; Lamb, J.R.; Law, B.; Gelling, V.J. Novel Synthesis of Stable Polypyrrole Nanospheres Using Ozone. Langmuir 2011, 27, 13719–13728. [Google Scholar] [CrossRef]
- Monfared, M.R.; Tavanai, H.; Abdolmaleki, A.; Morshed, M.; Shamsabadi, A.S. Fabrication of Polypyrrole nanoparticles through electrospraying. Mater. Res. Express 2019, 6, 0950c2. [Google Scholar] [CrossRef]
- Liu, J.; Wan, M. Studies on formation mechanism of polypyrrole microtubule synthesized by template free method. J. Polym. Sci. Pol. Chem. 2015, 39, 997–1004. [Google Scholar] [CrossRef]
- Turco, A.; Mazzotta, E.; Di Franco, C.; Santacroce, M.V.; Scamarcio, G.; Monteduro, A.G.; Primiceri, E.; Malitesta, C. Templateless synthesis of polypyrrole nanowires by non-static solution-surface electropolymerization. J. Solid State Electr. 2016, 20, 2143–2151. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Pan, L.; Yu, G. 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energ. Environ. Sci. 2013, 6, 2856–2870. [Google Scholar] [CrossRef]
- Attia, N.F.; Lee, S.M.; Kim, H.J.; Geckeler, K.E. Nanoporous polypyrrole: Preparation and hydrogen storage properties. Int. J. Energ. Res. 2014, 38, 466–476. [Google Scholar] [CrossRef]
- Ahn, K.J.; Lee, Y.; Choi, H.; Kim, M.S.; Im, K.; Noh, S.; Yoon, H. Surfactant-templated synthesis of polypyrrole nanocages as redox mediators for efficient energy storage. Sci. Rep. 2015, 5, 14097. [Google Scholar] [CrossRef] [Green Version]
- Devadas, B.; Imae, T. Effect of carbon dots on conducting polymers for energy storage applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Ko, J.; Im, S.S. Spherical polypyrrole nanoparticles as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 8146–8151. [Google Scholar] [CrossRef]
- Hwang, D.K.; Song, D.; Jeon, S.S.; Han, T.H.; Kang, Y.S.; Im, S.S. Ultrathin polypyrrole nanosheets doped with HCl as counter electrodes in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 859–865. [Google Scholar] [CrossRef]
- Peng, T.; Sun, W.; Huang, C.; Yu, W.; Sebo, B.; Dai, Z.; Guo, S.; Zhao, X. Self-assembled free-standing polypyrrole nanotube membrane as an efficient FTO- and Pt-free counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Inter. 2014, 6, 14–17. [Google Scholar] [CrossRef]
- Chang, L.; Li, C.; Li, Y.; Lee, C.P.; Yeh, M.H.; Ho, K.C.; Lin, J. Morphological influence of polypyrrole nanoparticles on the performance of dye–sensitized solar cells. Electrochim. Acta 2015, 155, 263–271. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Wen, Z.; Huang, L.; Wang, X.; Zhang, H. A nano-structured and highly ordered polypyrrole-sulfur cathode for lithium-sulfur batteries. J. Power Sources 2011, 196, 6951–6955. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Wang, Q.; Shen, C.; Peng, P.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Zou, Y.; Pisciotta, J.; Baskakov, I.V. Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochemistry 2010, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, S.; Li, Y.; Jiang, L.; Sun, H.; Zhu, S.; Su, D.; Sun, G. Vertically oriented polypyrrole nanowire arrays on Pd-plated Nafion (R) membrane and its application in direct methanol fuel cells. J. Mater. Chem. A 2013, 1, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Wang, S.; Jiang, L.; Sun, H.; Sun, G. Controllable synthesis of vertically aligned polypyrrole nanowires as advanced electrode support for fuel cells. J. Power Sources 2014, 256, 125–132. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, T.; Huang, Q.; Zhang, H.; Zhou, Z.; Zheng, J.; Yang, H. Polypyrrole nanowire networks as anodic micro-porous layer for passive direct methanol fuel cells. Electrochim. Acta 2014, 141, 1–5. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting Polymer Nanowire Arrays for High Performance Supercapacitors. Small 2014, 10, 14–31. [Google Scholar] [CrossRef]
- Dubal, D.P.; Patil, S.V.; Kim, W.B.; Lokhande, C.D. Supercapacitors based on electrochemically deposited polypyrrole nanobricks. Mater. Lett. 2011, 65, 2628–2631. [Google Scholar] [CrossRef]
- Dubal, D.P.; Patil, S.V.; Jagadale, A.D.; Lokhande, C.D. Two step novel chemical synthesis of polypyrrole nanoplates for supercapacitor application. J. Alloy Compd. 2011, 509, 8183–8188. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Shan, Q.; Wang, Z.; Wang, S. Tunable electrode morphology used for high performance supercapacitor: Polypyrrole nanomaterials as model materials. Electrochim. Acta 2013, 90, 535–541. [Google Scholar] [CrossRef]
- Dubal, D.P.; Lee, S.H.; Kim, J.G.; Kim, W.B.; Lokhande, C.D. Porous polypyrrole clusters prepared by electropolymerization for a high performance supercapacitor. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, P. Acid blue AS doped polypyrrole (PPy/AS) nanomaterials with different morphologies as electrode materials for supercapacitors. Chem. Eng. J. 2011, 172, 1137–1144. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Mousavi, M.F.; Rahmanifar, M.S. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta 2012, 78, 212–222. [Google Scholar] [CrossRef]
- Shen, C.; Sun, Y.; Yao, W.; Lu, Y. Facile synthesis of polypyrrole nanospheres and their carbonized products for potential application in high-performance supercapacitors. Polymer 2014, 55, 2817–2824. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, B.; Du, W.; Huang, Q.; Zheng, M.; Xue, H.; Pang, H. Facile synthesis of polypyrrole nanowires for high-performance supercapacitor electrode materials. Prog. Nat. Sci. 2016, 26, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, P.; Xu, Y.; Lin, J.; Li, H.; Bai, Y.; Zhu, J.; Mao, S.; Wang, J. Gravity-assisted synthesis of micro/nano-structured polypyrrole for supercapacitors. Chem. Eng. J. 2017, 330, 1060–1067. [Google Scholar] [CrossRef]
- Santino, L.M.; Acharya, S.; D’Arcy, J.M. Low-temperature vapour phase polymerized polypyrrole nanobrushes for supercapacitors. J. Mater. Chem. A 2017, 5, 11772–11780. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeong, H.; Lavall, R.L.; Busnaina, A.; Kim, Y.; Jung, Y.J.; Lee, H.Y. Polypyrrole films with micro/ nano sphere shapes for electrodes of high performance supercapacitors. ACS Appl. Mater. Inter. 2017, 9, 33203–33211. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Wei, C.; Xu, Q.; Chen, Z.; Rao, W.; Fan, L.; Yuan, Y.; Bai, Z.; Xu, J. An all-solid-state yarn supercapacitor using cotton yarn electrodes coated with polypyrrole nanotubes. Carbohydr. Polym. 2017, 169, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhou, Y.; Sun, H.; Zhang, G.; Wang, Y. Interconnected polypyrrole nanostructure for high-performance all-solid-state flexible supercapacitor. Electrochim. Acta 2019, 298, 918–923. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeon, S.H.; Seo, J.S.; Goh, S.H.; Han, J.Y.; Cho, Y. A novel strategy for highly efficient isolation and analysis of circulating tumor-specific cell-free DNA from lung cancer patients using a reusable conducting polymer nanostructure. Biomaterials 2016, 101, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Pokki, J.; Ergeneman, O.; Sivaraman, K.M.; Oezkale, B.; Zeeshan, M.A.; Luhmann, T.; Nelson, B.J.; Pane, S. Electroplated porous polypyrrole nanostructures patterned by colloidal lithography for drug-delivery applications. Nanoscale 2012, 4, 3083–3088. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Li, J.; Cirillo, J.; Borgens, R.; Cho, Y. Action at a distance: Functional drug delivery using electromagnetic-field-responsive polypyrrole nanowires. Langmuir 2014, 30, 7778–7788. [Google Scholar] [CrossRef]
- Samanta, D.; Meiser, J.L.; Zare, R.N. Polypyrrole nanoparticles for tunable, pH-sensitive and sustained drug release. Nanoscale 2015, 7, 9497–9504. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Borgens, R.B. Remote-controlled eradication of astrogliosis in spinal cord injury via electromagnetically–induced dexamethasone release from “smart” nanowires. J. Control. Release 2015, 211, 22–27. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Sun, Y.; Hu, Y.; Peng, Y.; Li, Y.; Yin, G.; Liu, H.; Xu, J.; Zhong, S. Synthesis of size-tunable hollow polypyrrole nanostructures and their assembly into folate-targeting and pH-responsive anticancer drug-delivery agents. Chem. Eur. J. 2017, 23, 17279–17289. [Google Scholar] [CrossRef]
- Samanta, D.; Hosseini-Nassab, N.; McCarthy, A.D.; Zare, R.N. Ultra-low voltage triggered release of an anti-cancer drug from polypyrrole nanoparticles. Nanoscale 2018, 10, 9773–9779. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Li, J.; Li, J.; Huang, L.; Ren, T.; Yang, X.; Zhong, S. Assembling of stimuli-responsive tumor targeting polypyrrole nanotubes drug carrier system for controlled release. Mat. Sci. Eng. C Mater. 2018, 89, 316–327. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, J.; Wu, C.; Zheng, Y.; Ye, C.; Huang, M.; Wang, S. One-pot synthesis of polypyrrole nanoparticles with tunable photothermal conversion and drug loading capacity. Colloid Surface B 2019, 177, 346–355. [Google Scholar] [CrossRef]
- Moquin, A.; Hanna, R.; Liang, T.; Erguven, H.; Gran, E.R.; Arndtsen, B.A.; Maysinger, D.; Kakkar, A. PEG-conjugated pyrrole-based polymers: One-pot multicomponent synthesis and self-assembly into soft nanoparticles for drug delivery. Chem. Commun. 2019, 55, 9829–9832. [Google Scholar] [CrossRef]
- Bernasconi, R.; Favara, N.; Turri, S.; Magagnin, L. Electrodeposited nanoporous polypyrrole layers for controlled drug release. J. Electrochem. Soc. 2019, 166, G122–G129. [Google Scholar] [CrossRef]
- Au, K.M.; Lu, Z.; Matcher, S.J.; Armes, S.P. Polypyrrole nanoparticles: A potential optical coherence tomography contrast agent for cancer imaging. Adv. Mater. 2011, 23, 5792–5795. [Google Scholar] [CrossRef]
- Chen, M.; Fang, X.; Tang, S.; Zheng, N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem. Commun. 2012, 48, 8934–8936. [Google Scholar] [CrossRef]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Qu, E.; Dai, Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, B.; Wang, C.; Lu, S.; Hu, X. In vivo photothermal inhibition of methicillin-resistant Staphylococcus aureus infection by in situ templated formulation of pathogen-targeting phototheranostics. Nanoscale 2020, 12, 7651–7659. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Qin, J.; Li, B.; Ye, K.; Zhang, Y.; Yang, X.; Yuan, F.; Huang, L.; Hu, J.; Lu, X. An effective approach to reduce inflammation and stenosis in carotid artery: Polypyrrole nanoparticle-based photothermal therapy. Nanoscale 2015, 7, 7682–7691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, H.; Wang, X.; Sun, S. Microwave-assisted ultrafast fabrication of high-performance polypyrrole nanoparticles for photothermal therapy of tumors in vivo. Biomater. Sci. 2018, 6, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderon, M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311, 147–161. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Tang, R. Spindle-like polypyrrole hollow nanocapsules as multifunctional platforms for highly effective chemo-photothermal combination therapy of cancer cells in vivo. Chem. Eur. J. 2014, 20, 11826–11834. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Tiwari, A.P.; Maharjan, B.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Sacrificial template-based synthetic approach of polypyrrole hollow fibers for photothermal therapy. J. Colloid Interf. Sci. 2018, 534, 447–458. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, S.; Wang, L.; Wang, M.; Wang, C.; Liu, S.; Zhang, K.; Yang, B. Hollow polypyrrole nanospindles for highly effective therapy of cancer. ChemPlusChem 2018, 83, 1127–1134. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin polypyrrole nanosheets via space-confined synthesis for efficient photothermal therapy in the second near-infrared window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef]
- Guan, H.; Ding, T.; Zhou, W.; Wang, Z.; Zhang, J.; Cai, K. Hexagonal polypyrrole nanosheets from interface driven heterogeneous hybridization and self-assembly for photothermal cancer treatment. Chem. Commun. 2019, 55, 4359–4362. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interf. Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Travas-Sejdic, J.; Aydemir, N.; Kannan, B.; Williams, D.E.; Malmstroem, J. Intrinsically conducting polymer nanowires for biosensing. J. Mater. Chem. B 2014, 2, 4593–4609. [Google Scholar] [CrossRef]
- Kwon, O.S.; Hong, T.J.; Kim, S.K.; Jeong, J.H.; Hahn, J.S.; Jang, J. Hsp90-functionalized polypyrrole nanotube FET sensor for anti-cancer agent detection. Biosens. Bioelectron. 2010, 25, 1307–1312. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar] [CrossRef]
- Huang, J.; Luo, X.; Lee, I.; Hu, Y.; Cui, X.T.; Yun, M.H. Rapid real-time electrical detection of proteins using single conducting polymer nanowire-based microfluidic aptasensor. Biosens. Bioelectron. 2011, 30, 306–309. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and selective recognition of peptide hormone using close-packed arrays of hPTHR-conjugated polymer nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef]
- Na, W.; Park, W.J.; An, J.H.; Jang, J. Size-controllable ultrathin carboxylated polypyrrole nanotube transducer for extremely sensitive 17 beta-estradiol FET-type biosensors. J. Mater. Chem. B 2016, 4, 5025–5034. [Google Scholar] [CrossRef]
- Tran, T.L.; Chu, T.X.; Huynh, D.C.; Pham, D.T.; Luu, T.H.T.; Mai, A.T. Effective immobilization of DNA for development of polypyrrole nanowires based biosensor. Appl. Surf. Sci. 2014, 314, 260–265. [Google Scholar] [CrossRef]
- Tuan, M.A.; Pham, D.T.; Chu, T.X.; Hieu, N.M.; Hai, N.H. Highly sensitive DNA sensor based on polypyrrole nanowire. Appl. Surf. Sci. 2014, 309, 285–289. [Google Scholar]
- Khoder, R.; Korri-Youssoufi, H. E-DNA biosensors of M. tuberculosis based on nanostructured polypyrrole. Mat. Sci. Eng. C Mater. 2020, 108, 110371. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Lin, L.; Liang, H.; Chen, X.; Han, C.; Li, J.; Yang, H. Polypyrrole nanoprobes with low non-specific protein adsorption for intracellular mRNA detection and photothermal therapy. Chem. Commun. 2015, 51, 6800–6803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hui, N. Electrochemical functionalization of polypyrrole nanowires for the development of ultrasensitive biosensors for detecting microRNA. Sensor Actuat. B Chem. 2019, 281, 478–485. [Google Scholar] [CrossRef]
- Lin, M.; Cho, M.; Choe, W.S.; Yoo, J.B.; Lee, Y. Polypyrrole nanowire modified with Gly-Gly-His tripeptide for electrochemical detection of copper ion. Biosens. Bioelectron. 2010, 26, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Hu, X.; Ma, Z.; Chen, L. Functionalized polypyrrole nanotube arrays as electrochemical biosensor for the determination of copper ions. Anal. Chim. Acta 2012, 746, 63–69. [Google Scholar] [CrossRef]
- Chartuprayoon, N.; Rheem, Y.; Ng, J.C.K.; Nam, J.; Chen, W.; Myung, N.V. Polypyrrole nanoribbon based chemiresistive immunosensors for viral plant pathogen detection. Anal. Methods 2013, 5, 3497–3502. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Palod, P.A.; Singh, V. Improvement in glucose biosensing response of electrochemically grown polypyrrole nanotubes by incorporating crosslinked glucose oxidase. Mat. Sci. Eng. C Mater. 2015, 55, 420–430. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.G.; Ren, J.S.; Qu, X. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 2014, 50, 3030–3032. [Google Scholar] [CrossRef]
- Tu, X.; Gao, Y.; Yue, R.; Lu, Q.; Zhou, Y.; Lu, Z. An amperometric nitrate sensor based on well-aligned cone-shaped polypyrrole-nanorods. Anal. Methods 2012, 4, 4182–4186. [Google Scholar] [CrossRef]
- Yang, X.; Li, L. Polypyrrole nanofibers synthesized via reactive template approach and their NH3 gas sensitivity. Synth. Met. 2010, 160, 1365–1367. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly sensitive and selective chemiresistive sensors based on multidimensional polypyrrole nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, B. A novel electropolymerization method for Ppy nanowire-based NH₃ gas sensor with low contact resistance. Sensor Actuat. B Chem. 2011, 160, 1168–1173. [Google Scholar] [CrossRef]
- Massafera, M.P.; de Torresi, S.I.C. Evaluating the performance of polypyrrole nanowires on the electrochemical sensing of ammonia in solution. J. Electroanal. Chem. 2012, 669, 90–94. [Google Scholar] [CrossRef]
- Ishpal; Kaur, A. Spectroscopic investigations of ammonia gas sensing mechanism in polypyrrole nanotubes/nanorods. J. Appl. Phys. 2013, 113, 094504. [Google Scholar] [CrossRef]
- Rawal, I.; Kaur, A. Synthesis of mesoporous polypyrrole nanowires/nanoparticles for ammonia gas sensing application. Sensor Actuat. A Phys. 2013, 203, 92–102. [Google Scholar] [CrossRef]
- Xue, M.; Li, F.; Wang, Y.; Cai, X.; Pan, F.; Chen, J. Ultralow-limit gas detection in nano-dumbbell polymer sensor via electrospinning. Nanoscale 2013, 5, 1803–1805. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Fang, D.; Wang, C.; Zhang, R.; Lu, X.; Li, Y.; Xu, W. Freestanding flexible polypyrrole nanotube membrane for ammonia sensor. Micro Nano Lett. 2018, 12, 997–999. [Google Scholar] [CrossRef]
- Alizadeh, N.; Babaei, M.; Nabavi, S. Gas sensing ability of a nanostructured conducting polypyrrole film prepared by catalytic electropolymerization on Cu/Au interdigital electrodes. Electroanalysis 2014, 25, 2181–2192. [Google Scholar] [CrossRef]
- Babaei, M.; Alizadeh, N. Methanol selective gas sensor based on nano-structured conducting polypyrrole prepared by electrochemically on interdigital electrodes for biodiesel analysis. Sensor Actuat. B Chem. 2013, 183, 617–626. [Google Scholar] [CrossRef]
- Jun, J.; Oh, J.; Shin, D.H.; Kim, S.G.; Lee, J.S.; Kim, W.; Jang, J. Wireless, room temperature volatile organic compound sensor based on polypyrrole nanoparticle immobilized ultrahigh frequency radio frequency identification tag. Acs Appl. Mater. Inter. 2016, 8, 33139–33147. [Google Scholar] [CrossRef] [PubMed]
- Shirale, D.J.; Bangar, M.A.; Chen, W.; Myung, N.V.; Mulchandani, A. Effect of aspect ratio (Length:Diameter) on a single polypyrrole nanowire FET device. J. Phys. Chem. C 2010, 114, 13375–13380. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashat, L.; Debiemme-Chouvy, C.; Borensztajn, S.; Wlodarski, W. Electropolymerized polypyrrole nanowires for hydrogen gas sensing. J. Phys. Chem. C 2012, 116, 13388–13394. [Google Scholar] [CrossRef]
- Guan, G.; Wang, S.; Zhou, H.; Zhang, K.; Liu, R.; Mei, Q.; Wang, S.; Zhang, Z. Molecularly imprinted polypyrrole nanonecklaces for detection of herbicide through molecular recognition-amplifying current response. Anal. Chim. Acta 2011, 702, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. Distinctive polypyrrole nanobelts as prospective electrode for the direct detection of aliphatic alcohols: Electrocatalytic properties. Appl. Catal. B Environ. 2014, 144, 665–673. [Google Scholar] [CrossRef]
- Xu, T.; Dai, H.; Jin, Y. Electrochemical sensing of lead (II) by differential pulse voltammetry using conductive polypyrrole nanoparticles. Microchim. Acta 2020, 187, 23. [Google Scholar] [CrossRef]
- Venugopal, V.; Venkatesh, V.; Northcutt, R.G.; Maddox, J.; Sundaresan, V.B. Nanoscale polypyrrole sensors for near-field electrochemical measurements. Sensor Actuat. B Chem. 2017, 242, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Kim, S.G.; Jun, J.; Shin, D.H.; Jang, J. Aptamer-functionalized multidimensional conducting-polymer nanoparticles for an ultrasensitive and selective field-effect-transistor endocrine-disruptor sensors. Adv. Funct. Mater. 2014, 24, 6145–6153. [Google Scholar] [CrossRef]
- Yao, T.; Cui, T.; Wu, J.; Chen, Q.; Lu, S.; Sun, K. Preparation of hierarchical porous polypyrrole nanoclusters and their application for removal of Cr (VI) ions in aqueous solution. Polym. Chem. 2011, 2, 2893–2899. [Google Scholar] [CrossRef]
- Mondal, P.; Roy, K.; Bayen, S.P.; Chowdhury, P. Synthesis of polypyrrole nanoparticles and its grafting with silica gel for selective binding of chromium (VI). Talanta 2011, 83, 1482–1486. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Li, X.; Xue, Y.; Zhang, C.; Lei, J.; Wang, C. Preparation of bamboo-like PPy nanotubes and their application for removal of Cr(VI) ions in aqueous solution. J. Colloid Interf. Sci. 2012, 378, 30–35. [Google Scholar] [CrossRef]
- Hosseini, S.; Mahmud, N.H.M.E.; Yahya, R.B.; Ibrahim, F.; Djordjevic, I. Polypyrrole conducting polymer and its application in removal of copper ions from aqueous solution. Mater. Lett. 2015, 149, 77–80. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Lv, W.; Xu, H.; Yang, H.; Yan, W. Synthesis of polypyrrole nano-fibers with hierarchical structure and its adsorption property of Acid Red G from aqueous solution. Synth. Met. 2014, 191, 66–73. [Google Scholar] [CrossRef]
- Xin, Q.; Fu, J.; Chen, Z.; Liu, S.; Yan, Y.; Zhang, J.; Xu, Q. Polypyrrole nanofibers as a high-efficient adsorbent for the removal of methyl orange from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1637–1647. [Google Scholar] [CrossRef]
- Wong, P.T.C.; Chambers, B.; Anderson, A.P.; Wright, P.V. Large area conducting polymer composites and their use in microwave absorbing material. Electron. Lett. 1992, 28, 1651–1653. [Google Scholar] [CrossRef]
- Kaur, A.; Ishpal; Dhawan, S.K. Tuning of EMI shielding properties of polypyrrole nanoparticles with surfactant concentration. Synth. Met. 2012, 162, 1471–1477. [Google Scholar] [CrossRef]
- Xie, A.; Wu, F.; Jiang, W.; Zhang, K.; Sun, M.; Wang, M. Chiral induced synthesis of helical polypyrrole (PPy) nano-structures: A lightweight and high-performance material against electromagnetic pollution. J. Mater. Chem. C 2017, 5, 2175–2181. [Google Scholar] [CrossRef]
- Wu, J.; Lord, H.L.; Pawliszyn, J.; Kataoka, H. Polypyrrole-coated capillary in-tube solid phase microextraction coupled with liquid chromatography-electrospray ionization mass spectrometry for the determination of β-blockers in urine and serum samples. J. Microcolumn Sep. 2000, 12, 255–266. [Google Scholar] [CrossRef]
- Wu, J.; Pawliszyn, J. Polypyrrole-coated capillary coupled to hplc for in-tube solid phase microextraction and analysis of aromatic compounds in aqueous samples. Anal. Chem. 2001, 73, 55–63. [Google Scholar] [CrossRef]
- Wu, J.; Lord, H.; Pawliszyn, J. Determination of stimulants in human urine and hair samples by polypyrrole coated capillary in-tube solid phase microextraction coupled with liquid chromatography electrospray mass spectrometry. Talanta 2001, 54, 655–672. [Google Scholar] [CrossRef]
- Mehdinia, A.; Bashour, F.; Roohi, F.; Jabbari, A. A strategy to enhance the thermal stability of a nanostructured polypyrrole-based coating for solid phase microextraction. Microchim. Acta 2012, 177, 301–308. [Google Scholar] [CrossRef]
- Kalhor, H.; Ameli, A.; Alizadeh, N. Electrochemically controlled solid-phase micro-extraction of proline using a nanostructured film of polypyrrole, and its determination by ion mobility spectrometry. Microchim. Acta 2013, 180, 783–789. [Google Scholar] [CrossRef]
- Mohammadkhani, E.; Yamini, Y.; Rezazadeh, M.; Seidi, S. Electromembrane surrounded solid phase microextraction using electrochemically synthesized nanostructured polypyrrole fiber. J. Chromatogr. A 2016, 1443, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Kamalabadi, M.; Mohammadi, A. Determination of histamine and tyramine in canned fish samples by headspace solid-phase microextraction based on a nanostructured polypyrrole fiber followed by ion mobility spectrometry. Food Anal. Method 2017, 10, 3001–3008. [Google Scholar] [CrossRef]
- de Lazzari, A.C.; Soares, D.P.; Sampaio, N.M.F.M.; Silva, B.J.G.; Vidotti, M. Polypyrrole nanotubes for electrochemically controlled extraction of atrazine, caffeine and progesterone. Microchim. Acta 2019, 186, 398. [Google Scholar] [CrossRef]
- Xie, L.; Huang, J.; Han, Q.; Song, Y.; Liu, P.; Kang, X. Solid phase extraction with Polypyrrole nanofibers for simultaneously determination of three water-soluble vitamins in urine. J. Chromatogr. A 2019, 1589, 30–38. [Google Scholar] [CrossRef]
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated polymer actuators and devices: Progress and opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef]
- Proennecke, C.; Staude, M.; Frank, R.; Jahnke, H.G.; Robitzki, A.A. Electrically switchable monostable actuatoric polymer-based nanovalve arrays with a long-term stability. Nano Lett. 2018, 18, 6375–6380. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Zhang, S.; Xu, H.; Ding, T. Light-controlled nanoswitches: From fabrication to photoelectric switching. Nanoscale 2019, 11, 18496–18500. [Google Scholar] [CrossRef]
- Liao, J.; Zhu, Y.; Zhou, Z.; Chen, J.; Tan, G.; Ning, C.; Mao, C. Reversibly controlling preferential protein adsorption on bone implants by using an applied weak potential as a switch. Angew. Chem. Int. Edit. 2014, 53, 13068–13072. [Google Scholar] [CrossRef] [Green Version]
- Ghoorchian, A.; Tavoli, F.; Alizadeh, N. Long-term stability of nanostructured polypyrrole electrochromic devices by using deep eutectic solvents. J. Electroanal. Chem. 2017, 807, 70–75. [Google Scholar] [CrossRef]
- Knittel, P.; Higgins, M.J.; Kranz, C. Nanoscopic polypyrrole AFM-SECM probes enabling force measurements under potential control. Nanoscale 2014, 6, 2255–2260. [Google Scholar] [CrossRef]
- Cui, X.; Huang, X.; He, Y.; Dai, L.; Wang, S.; Sun, Y.; Shi, W.; Ge, D. Cathodic protection: A new strategy to enable the formation of nanostructured polypyrrole on magnesium. Synth. Met. 2014, 195, 97–101. [Google Scholar] [CrossRef]
- Teng, C.; Wang, S.; Lu, X.; Wang, J.; Ren, G.; Zhu, Y.; Jiang, L. Stable underwater superoleophobic and low adhesive polypyrrole nanowire mesh in highly corrosive environments. Soft Matter 2015, 11, 4290–4294. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, J.; Zhang, Y.; Tian, X.; Chen, W. Dispersed conductive polymer nanoparticles on graphitic carbon nitride for enhanced solar-driven hydrogen evolution from pure water. Nanoscale 2013, 5, 9150–9155. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- de Morais, V.B.; Correa, C.C.; Lanzoni, E.M.; Costa, C.A.R.; Bufon, C.C.B.; Santhiago, M. Wearable binary cooperative polypyrrole nanofilms for chemical mapping on the skin. J. Mater. Chem. A 2019, 7, 5227–5233. [Google Scholar] [CrossRef]
- Weng, B.; Shepherd, R.; Chen, J.; Wallace, G.G. Gemini surfactant doped polypyrrole nanodispersions: An inkjet printable formulation. J. Mater. Chem. 2011, 21, 1918–1924. [Google Scholar] [CrossRef]










| Morphology | Surfactant/Template | Dopant | Oxidant | Diameter (nm) | Conductivity (25 °C, S/cm) | Ref. |
|---|---|---|---|---|---|---|
| Spheres | Castor oil sulfate | Castor oil sulfate | (NH4)2S2O8 | 20–100 | 1–6 | [7] |
| Spheres | PVP | FeCl3 | 30–60 | 10–15 | [8] | |
| Particles | CTAB | HCl | (NH4)2S2O8 | 20 | [9] | |
| Particles | Polyoxyethylene nonylphenyl ether sulfate | Polyoxyethylene nonylphenyl ether sulfate | (NH4)2S2O8 | 40–150 | 1–20 | [10] |
| Particles | SDS | FeCl3 | 28–52 | 3–22 | [11] | |
| Spheres | Fatty alcohol–polyoxyethylene ethers | (NH4)2S2O8 | 60–230 | [12] | ||
| Particles | Sodium taurocholate and Tween 20 | Citric acid | H2O2 | 100–500 | 10−2 | [13] |
| Spheres | Iodine | 35–350 | 10−7–10−4 | [14] | ||
| Spheres | Heparin | 50–80 | [17] | |||
| Irregular | Methyl red | Methyl red | FeCl3 | 84 | [19] | |
| Spheres | Pluronics® F-108 | Formic acid | (NH4)2S2O8 | 110–120 | [21] | |
| Particles | PVA | FeCl3 | 20–60 | 0–10 | [23] | |
| Particles | Poly (ethylene glycol) (PEG) | Organic sulfonic acid | (NH4)2S2O8 | 71–134 | 3.26–52.7 | [24] |
| Particles | SDS | HCl | H2O2 | 28 | [25] | |
| Particles | PVP | H2SO4 | H2O2 | 21–92 | 7.67 × 10−3 | [26] |
| Particles | PVA | FeCl3 | 20–100 | [28] | ||
| Particles | PVA | FeCl3 | 20–80 | 1.71–149.08 | [30] | |
| Particles | PVA | FeCl3 | 20–100 | 1–10 | [31] | |
| Double-walled shells | Au nanocages | PVP | FeCl3 | 5 nm (two shells spacing) | [32] | |
| Urchin-like particles | PVA | FeCl3 | 30, 60, 100 | [33] | ||
| Hollow spheres | Poly (methyl methacrylate) | FeCl3 | 136.5 (inner) and 242 (outer) | [34] | ||
| Double-shelled hollow particles | Polystyrene | PVP | FeCl3 | [35] | ||
| Bowl-shaped particles | Iodine | FeCl3 | [36] |
| Method | Morphology | Reaction Medium | Oxidant | Properties | Ref. |
|---|---|---|---|---|---|
| Hard template/Electropolymerization | Nanowire arrays | A 0.1 M LiClO4 solution | A good potentiometric response to pH changes and a very good stability in time | [81] | |
| Soft template/ Chemical oxidation | Nanoscale hierarchical structure | Aqueous solution | FeCl3 | A high specific capacitance and good electrochemical reversibility | [104] |
| Soft template/Electropolymerization | Nanostructured membranes | Milli Q water | A high specific surface area and high specific capacitance | [105] | |
| Soft template/Chemical oxidation | Nanoparticles | Aqueous solution | FeCl3 | Potentially useful to detect acetone | [106] |
| Soft template/Interfacial polymerization | Nanowires | Organic/aqueous interface | (NH4)2S2O8 | A high specific capacitance | [107] |
| Hard template/Chemical oxidation | Nanotubes | Ethanol solution | FeCl3 | Undergo a spontaneous redox reaction with metal ions | [109] |
| Hard template/Chemical oxidation | Nanofibers | Aqueous solution | H2O2 | Bulk quantities | [111] |
| Hard template/ In-situ vapor phase polymerization | Hollow nanofibers | In desiccators | A high Cr (VI) adsorption capacity up to 839.3 mg g−1 | [112] | |
| Hard template/Electropolymerization | Nanopore arrays | An ionic-surfactant-solution | Forming mechanically stable and underlying compact films | [113] | |
| Hard template/Electropolymerization | Nanowires and nanopore arrays | Electrolyte of dodecyl sulfate | Well-organized and mechanically stable | [114] | |
| Hard template/Chemical oxidation | Nanofibers | Aqueous solution | K2Cr2O7 | Enhanced electroactive surface area | [115] |
| Hard template/Electropolymerization | Nanotube arrays | CH2Cl2 or acetonitrile solution | A facile, inexpensive and large-scale means for generating polymeric nanostructures | [116] | |
| Template-free/Electropolymerization | Hollow “horns” in nanometers | P-toluenesulfonate alkaline solution | High specific surface area and high ionic and electronic conductivity | [117] | |
| Template-free/Electropolymerization | Nanotube arrays | Phosphate buffer solution | Enhanced electrical and electrochemical performances | [118] | |
| Template-free/Electropolymerization | Nano-snails | Aqueous alkaline solution | Fe (CN)63− | Promising potential applications in supercapacitors and sensors | [120] |
| Template-free/electropolymerization | Nanowires | A 70:30 H2O/EtOH mixture | Forming a uniform polymer film | [122] | |
| Template-free/electrohydrodynamic lithography | Nanostructured films | Aqueous solution | (NH4)2S2O8 | Accessing scale sizes in the low submicron range | [123] |
| Template-free/electrochemical lithography | Nanostructured films | Aqueous solution | A reversible, erasable, and rewritable pattern | [124] | |
| Template-free/edge nanoimprint lithography | Nanowires | Aqueous solution | FeCl3 | Exhibiting representative ohmic behavior and excellent sensitivity to NH3 | [125] |
| Template-free/γ-radiation-induced chemical oxidative | Polydisperse spherical nanoparticles | Aqueous solution | K2S2O8 | Well dispersed in water, easily dried and quite simply redispersed in protic solvents | [127] |
| Template-free/mechanochemical route | Nanospheres | Pre-cleaned mortar | K2S2O8 | High degree of processability, electrochemical activity and film forming ability | [128] |
| Template-free/chemical oxidation | Nanospheres | Aqueous solution | O3 | Stable and unagglomerated | [129] |
| Template-free/electropolymerization | Nanowires | Acetonitrile solution | Fe (CN)63− | Low cost, simplicity, rapidity, and versatility | [131] |
| Morphology | Analyte | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| Nanoparticles | Acetone | 5.5–80 ppm | 5.5 ppm | [106] |
| Nanotubes | Heat shock protein 90 inhibitors | 40 nM–8 μM | 40 nM | [194] |
| Nanotubes | Vascular Endothelial Growth Factor | 400 fM–4 μM | 400 fM | [195] |
| Nanowires | IgE protein | 0.01–100 nM | 0.01 nM | [196] |
| Nanoparticles | Peptide hormones | 48 fM–48 pM | 48 fM | [197] |
| Nanotubes | 17β-estradiol | 1 fM–1 nM | 1 fM | [198] |
| Nanowires | DNA | 10 pM–500 nM | 10 pM | [199] |
| Nanowires | Escherichia coli DNA | 0.1 nM | [200] | |
| Nanowires | DNA | 1 aM–100 fM | 0.36 aM | [201] |
| Nanowires | microRNA | 0.1 pM–1 nM | 0.033 pM | [203] |
| Nanowires | Cu2+ | 20–300 nM | 20 nM | [204] |
| Nanotube arrays | Cu2+ | 0.1–30 μM | 46 nM | [205] |
| Nanoribbons | Viral plant pathogen | 10 ng ml−1–100 μg ml−1 | 10 ng ml−1 | [206] |
| Films | SARS-CoV-2-S glycoprotein | 0–25 μg ml−1 | 0.15 μg ml−1 | [207] |
| Nanotube arrays | Glucose | 0.2–13 mM | 50 mu M | [208] |
| Nanoparticles | H2O2 | 5–100 μM | 5 μM | [209] |
| Nanorods | Nitrate | 1.0 × 10−4–5.0 × 10−3 mol L−1 | 5.0 × 10−5 mol L−1 | [210] |
| Nanotubes | NH3 | 0.01 ppm | [212] | |
| Nanowires | NH3 | 1–100 ppm | 0.4 ppm | [213] |
| Nano-dumbbells | NH3 | 1 ppb–1 ppm | 1 ppb | [217] |
| Nanoparticles | NH3, acetic acid | 1–100 ppm | 0.1 ppm, 1 ppm | [221] |
| Nanowires | H2 | 600–2500 ppm | 12 ppm | [223] |
| Nanonecklaces | 2,4-dichlorophenoxyacetic acid | 0.1–8 μM | 100 nM | [224] |
| Nanobelts | Methanol | 20 μM–0.16 mM | 6.92 μM | [225] |
| Nanoparticles | Pb2+ | 0.1–50 μM | 55 nM | [226] |
| Nanoparticles | Bisphenol A | 1–104 fM | 1 f M | [228] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Dong, C.; Zhang, L.; Zhu, K.; Yu, D. Polypyrrole Nanomaterials: Structure, Preparation and Application. Polymers 2022, 14, 5139. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14235139
Hao L, Dong C, Zhang L, Zhu K, Yu D. Polypyrrole Nanomaterials: Structure, Preparation and Application. Polymers. 2022; 14(23):5139. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14235139
Chicago/Turabian StyleHao, Lu, Changyi Dong, Lifeng Zhang, Kaiming Zhu, and Demei Yu. 2022. "Polypyrrole Nanomaterials: Structure, Preparation and Application" Polymers 14, no. 23: 5139. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14235139