Colorful Luminescence of Conjugated Polyelectrolytes Induced by Molecular Weight
Abstract
:1. Introduction
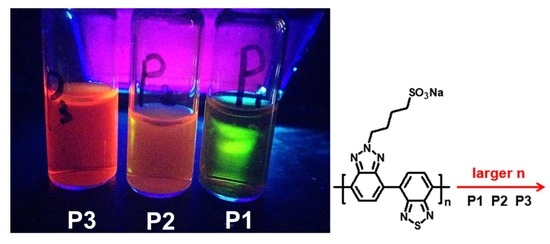
2. Results and Discussions
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, A.R.; Minckler, E.D.; Shockley, M.C.J.; Matsushima, L.N.; Perry, S.L.; Ayzner, A.L. Conjugated Polyelectrolyte-Based Complex Fluids as Aqueous Exciton Transport Networks. Angew. Chem. Int. Ed. 2022, 61, e202117759. [Google Scholar] [CrossRef]
- Korley, L.T.J.; Epps, T.H., III; Helms, B.A.; Ryan, A.J. Toward polymer upcycling-adding value and tackling circularity. Science 2021, 373, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Karst, J.; Floess, M.; Dingler, M.C.; Malacrida, C.; Steinle, T.; Ludwigs, S.; Hentschel, M.; Giessen, H. Electrically switchable metallic polymer nanoantennas. Science 2021, 374, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Y.; Gao, J.; Zhang, L.; Ma, Z.; Liu, M.; Emrick, T.; Liu, Y. Synchronous Doping Effects if Cathode Interlayers on Efficient Organic Solar Cells. ACS Energy Lett. 2022, 7, 4052–4060. [Google Scholar] [CrossRef]
- Jiang, Y.; Dong, X.; Sun, L.; Liu, T.; Qin, F.; Xie, C.; Jiang, P.; Hu, L.; Lu, X.; Zhou, X.; et al. An alcohol-dispersed conducting polymer complex for fully printable organic solar cells with improved stability. Nat. Energy 2022, 7, 352–359. [Google Scholar] [CrossRef]
- Margossian, K.O.; Brown, M.U.; Emrick, T.; Muthukumar, M. Coacervation in polyzwitterion-polyelectrolyte systems and their potential applications for gastrointestinal drug delivery platforms. Nat. Commun. 2022, 13, 2250. [Google Scholar] [CrossRef]
- Besford, Q.A.; Yong, H.; Merlitz, H.; Christofferson, A.J.; Sommer, J.-U.; Uhlmann, P.; Fery, A. FRET-integrated polymer brushes for spatially resolved sensing of changes in polymer conformation. Angew. Chem. Int. Ed. 2021, 60, 16600–16606. [Google Scholar] [CrossRef]
- Hlaváček, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horák, D.; Skládal, P.; Foret, F.; Gorris, H.H. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef]
- Ishii, Y.; Matubayasi, N.; Watanabe, G.; Kato, T.; Washizu, H. Molecular insights on confined water in the nanochannels of self-assembled ionic liquid crystal. Sci. Adv. 2021, 7, eabf0669. [Google Scholar] [CrossRef]
- Tsokkou, D.; Peterhans, L.; Cao, D.X.; Mai, C.-K.; Bazan, G.C.; Nguyen, T.-Q.; Banerji, N. Excited State Dynamics of a Self-Doped Conjugated Polyelectrolyte. Adv. Funct. Mater. 2020, 30, 1906148. [Google Scholar] [CrossRef]
- Friedowitz, S.; Lou, J.; Barker, K.P.; Will, K.; Xia, Y.; Qin, J. Looping-in complexation and ion partitioning in nonstoichiometric polyelectrolyte mixtures. Sci. Adv. 2021, 7, eabg8654. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Lu, S.; Wang, Z.; Liu, S.; Yu, X.; Li, X.; Sun, Y. Construction of emissive ruthenium (II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nat. Commun. 2022, 13, 2009. [Google Scholar] [CrossRef] [PubMed]
- Au, B.W.-C.; Chan, K.-Y. Towards an All-Solid-State Electrochromic Device: A Review of Solid-State Electrolytes and the Way Forward. Polymers 2022, 14, 2458. [Google Scholar] [CrossRef]
- Armin, A.; Juska, G.; Philippa, B.W.; Burn, P.L.; Meredith, P. Doping-induced screening of the built-in-field in organic solar cells: Effect on charge transport and recombination. Adv. Energy Mater. 2013, 3, 321–327. [Google Scholar] [CrossRef]
- Bin, H.; Gao, L.; Zhang, Z.G.; Yang, Y.; Zhang, Y. 11.4% efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 2016, 7, 13651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Jiang, K.; Yang, G.; Lai, J.Y.L.; Ma, T. Donor polymer design enables efficient non-fullerene organic solar cells. Nat. Commun. 2016, 7, 13094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Q.; Bazan, G.C. Narrow band gap conjugated polyelectrolytes. Acc. Chem. Res. 2018, 51, 202–211. [Google Scholar] [CrossRef]
- Osaka, I.; Zhang, R.; Sauvé, G.; Smilgies, D.-M.; Kowalewski, T.; McCullough, R.D. High-lamellar Ordering and Amorphous-Like π-Network in Short-Chain Thiazolothiazole-Thiophene Copolymers Lead to High Mobilities. J. Am. Chem. Soc. 2015, 131, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Sohaimy, M.I.H.M.; Isa, I.N. Proton-Conduction Biopolymer Electrolytes Based on Carboxymethyl Cellulose Doped with Ammonium Formate. Polymers 2022, 14, 3019. [Google Scholar] [CrossRef]
- Baidurah, S. Methods of Analyses for Biodegradable Polymers: A Review. Polymers 2022, 14, 4928. [Google Scholar] [CrossRef]
- Shi, Y.; Mai, C.K.; Fronk, S.L.; Chen, Y.; Bazan, G.C. Optical properties of benzotriazole-based conjugated polyelectrolytes. Macromolecules 2016, 49, 6343–6349. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.S.; Frampton, M.J.; Michels, J.J.; Sardone, L.; Marletta, G.; Friend, R.H.; Samorì, P.; Anderson, H.L.; Cacialli, F. Supramolecular Complexes of Conjugated Polyelectrolytes with Poly(ethylene oxide): Multifunctional Luminescent Semiconductors Exhibiting Electronic and Ionic Transport. Adv. Mater. 2005, 17, 2659–2663. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, L.; Chen, Y. Dye-sensitized nanoarrays with discotic liquid crystals as interlayer for high-efficiency inverted polymer solar cells. ACS Appl. Mater. Interface 2014, 6, 17848–17856. [Google Scholar] [CrossRef] [PubMed]
- Fronk, S.L.; Shi, Y.; Siefrid, M.; Mai, C.K.; McDowell, C.; Bazan, G.C. Chiroptical properties of a benzotriazole-thiophene copolymer bearing chiral ethylhexyl side chains. Macromolecules 2016, 49, 9301–9308. [Google Scholar] [CrossRef]
- Peet, J.; Cho, N.S.; Lee, S.K.; Bazan, G.C. Transition from solution to the solid state in polymer solar cells cast from mixed solvents. Macromolecules 2008, 41, 8655–8659. [Google Scholar] [CrossRef]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Qian, X.; Zhu, W.; Li, B.; Zhang, B.; Lu, W.; Li, R.; Zhang, S.; Zhu, L.; et al. Relaxor ferroelectric polymer exhibits ultrahigh electromechanical coupling at low electric field. Science 2022, 375, 1418–1422. [Google Scholar] [CrossRef]
- Wade, J.; Hilfiker, J.N.; Brandt, J.R.; Liirò-Peluso, L.; Wan, L.; Shi, X.; Salerno, F.; Ryan, S.T.J.; Schöche, S.; Arteaga, O.; et al. Natural optical activity as the origin of the large chiroptical properties in π-conjugated polymer thin films. Nat. Commun. 2020, 11, 6137. [Google Scholar] [CrossRef]
- Fu, H.; Yao, J.; Zhang, M.; Xue, L.; Zhou, Q.; Li, S.; Lei, M.; Meng, L.; Zhang, Z.-G.; Li, Y. Low-cost synthesis of small molecule acceptors makes polymer solar cells commercially viable. Nat. Commun. 2022, 13, 3687. [Google Scholar] [CrossRef]
- Qi, F.; Jiang, K.; Lin, F.; Wu, Z.; Zhang, H.; Gao, W.; Li, Y.; Cai, Z.; Woo, H.Y.; Zhu, Z.; et al. Over 17% Efficiency Binary Organic Solar Cells with Photoresponses Reaching 1000 nm Enabled by Selenophene-Fused Nonfullerene Acceptors. ACS Energy Lett. 2021, 6, 826. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; He, Z.; Cao, Y. Formation Mechanism of PFN Dipole Interlayer in Organic Solar Cells. Sol. RRL 2021, 5, 2000753. [Google Scholar] [CrossRef]
- Ma, Q.; Jia, Z.; Meng, L.; Zhang, J.; Zhang, H.; Huang, W.; Yuan, J.; Gao, F.; Wan, Y.; Zhang, Z.; et al. Promoting charge separation resulting in ternary organic solar cells efficiency over 17.5%. Nano Energy 2020, 78, 105272. [Google Scholar] [CrossRef]
- Tybrandt, K.; Zozoulenko, I.V.; Berggren, M. Chemical potential-electric double layer coupling in conjugated polymer-polyelectrolyte blends. Sci. Adv. 2017, 3, eaao3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Raynold, A.A.M.; Li, D.; Chang, L.; Gautrot, J.E. Competitive binding and molecular crowding regulate the cytoplasmic interactome of non-viral polymeric gene delivery vectors. Nat. Commun. 2021, 12, 6445. [Google Scholar] [CrossRef]
- Liao, P.; Zang, S.; Wu, T.; Jin, H.; Wang, W.; Huang, J.; Tang, B.Z.; Yan, Y. Generating circularly polarized luminescence from clusterization-triggerd emission using solid phase molecular self-assembly. Nat. Commun. 2021, 12, 5496. [Google Scholar] [CrossRef]
- Wan, Y.; Ramirez, F.; Zhang, X.; Nguyen, T.-Q.; Bazan, G.C.; Lu, G. Data driven discovery of conjugated polyelectrolytes for optoelectronic and photocatalytic applications. NPJ Comput. Mater. 2021, 7, 69. [Google Scholar] [CrossRef]
- Tan, C.; Pinto, M.R.; Kose, M.E.; Ghiviriga, I.; Schanze, K.S. Solvent-induced self-assembly of a meta-linked conjugated polyelectrolyte. Helix formation, guest intercalation and amplified quenching. Adv. Mater. 2004, 16, 1208–1211. [Google Scholar] [CrossRef]
- Chambers, J.E.; Zubkov, N.; KubÁnkovÁ, M.; Nixon-Abell, J.; Mela, I.; Abreu, S.; Schwiening, M.; Lavarda, G.; López-Duarte, I.; Kuimova, M.K.; et al. Z-α1-antitrypsin polymers impose molecular filtration in the endoplasmic reticulum after undergoing phase transition to a solid state. Sci. Adv. 2022, 8, eabm2094. [Google Scholar] [CrossRef]
- Cha, H.; Wheeler, S.; Holliday, S.; Dimitrov, S.D.; Wadsworth, A.; Lee, H.H.; Baran, D.; McCulloch, I.; Durrant, J.R. Influence of blend morphology and energetics on charge separation and recombination dynamics in organic solar cells incorporating a nonfullerene acceptor. Adv. Funct. Mater. 2018, 28, 1704389. [Google Scholar] [CrossRef]
- Lahiri, S.; Thompson, J.L.; Moore, J.S. Solvophobically driven π-stacking of phenylene ethynylene macrocycles and oligomers. J. Am. Chem. Soc. 2000, 122, 11315–11319. [Google Scholar] [CrossRef]
- Nguyen-Dang, T.; Chae, S.; Chatsirisupachai, J.; Wakidi, H.; Promarak, V.; Visell, Y.; Nguyen, T.-Q. Dual-mode organic electrochemical transistors based on self-doped conjugated polyelectrolytes for reconfigurable electronics. Adv. Mater. 2022, 34, 2200274. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, C.; Yap, B.K.; Zhu, X.; Xiong, B.; He, Z.; Wong, W.-Y. Accelerating charge transfer via nonconjugated polyelectrolyte interlayers toward efficient versatile photoredox catalysis. Commun. Chem. 2021, 4, 150. [Google Scholar] [CrossRef]
- Koh, Q.-M.; Tang, C.G.; Ang, M.C.-Y.; Choo, K.-K.; Seah, Q.-J.; Png, R.-Q.; Chua, L.-L.; Ho, P.K.H. Overcoming the water oxidative limit for ultra-high-workfunction hole-doped polymers. Nat. Commun. 2021, 12, 3345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Liao, W.-Q. Giant electromechanical effects in polymers. Science 2022, 375, 1353–1354. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, J. Thermoresponsive PEDOT:PSS/PNIPAM conductive hydrogels as wearable resistive sensors for breathing pattern detection. Poly. J. 2022, 54, 793–801. [Google Scholar] [CrossRef]
- Morrls, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence Quantum Yield Determinations. 9,10-Diphenylanthracene as A Reference Standard in Different Solvents. J. Phys. Chem. 1976, 80, 969–974. [Google Scholar] [CrossRef]






| Polymers | Solution | Film | ||
|---|---|---|---|---|
| λEX2 (nm) | λEX1 (nm) | λEX2 (nm) | λEX3 (nm) | |
| P1 | 440 | 445 | 469 | \ |
| P2 | 461 | 451 | 476 | 517 |
| P3 | 443 | 444 | 481 | 515 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shi, Y.; Li, Z. Colorful Luminescence of Conjugated Polyelectrolytes Induced by Molecular Weight. Polymers 2022, 14, 5372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14245372
Wang K, Shi Y, Li Z. Colorful Luminescence of Conjugated Polyelectrolytes Induced by Molecular Weight. Polymers. 2022; 14(24):5372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14245372
Chicago/Turabian StyleWang, Kunsheng, Yueqin Shi, and Zhengjun Li. 2022. "Colorful Luminescence of Conjugated Polyelectrolytes Induced by Molecular Weight" Polymers 14, no. 24: 5372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14245372