Defect Passivation Using Trichloromelamine for Highly Efficient and Stable Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NiOx Nanoparticles
2.2. Materials and Preparation of Solutions
2.3. Preparation for Characterization
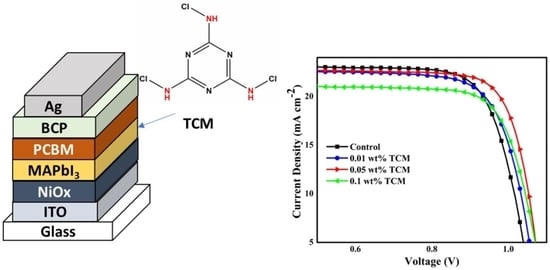
2.4. Device Fabrication
2.5. Characterization
3. Results
3.1. Film Properties
3.2. Charge Carrier Dynamic
3.3. Device Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Yang, Y.M.; Song, T.B.; Chen, C.C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef] [Green Version]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Ed. Engl. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Rao, H.; Wei, C.; Liu, Z.; Bian, Z.; Xin, H.; Huang, W. Highly efficient and stable inverted planar solar cells from (FAI)x(MABr)1−xPbI2 perovskites. Nano Energy 2017, 35, 62–70. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, X.; Qi, F.; Li, Z.; Liu, D.; Shen, D.; Qin, M.; Wu, S.; Lin, F.; Jang, S.-H. Regulating surface termination for efficient inverted perovskite solar cells with greater than 23% efficiency. J. Am. Chem. Soc. 2020, 142, 20134–20142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Wang, S.; Wu, Y.; Yuan, N.; Zhang, W.-H. Interfacial contact passivation for efficient and stable cesium-formamidinium double-cation lead halide perovskite solar cells. Iscience 2020, 23, 100762. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhan, Y.; Xu, G.; Chen, W.; Wang, S.; Zhang, M.; Li, Y.; Li, Y. Organic N-Type Molecule: Managing the Electronic States of Bulk Perovskite for High-Performance Photovoltaics. Adv. Funct. Mater. 2020, 30, 2001788. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.-C.; Lee, S.-J.; Wang, G.; Feng, Z.; Goddard III, W.A. Layer-by-layer degradation of methylammonium lead tri-iodide perovskite microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, X.; You, J. Recent progresses on defect passivation toward efficient perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902650. [Google Scholar] [CrossRef]
- Hassan, Y.; Park, J.H.; Crawford, M.L.; Sadhanala, A.; Lee, J.; Sadighian, J.C.; Mosconi, E.; Shivanna, R.; Radicchi, E.; Jeong, M. Ligand-engineered bandgap stability in mixed-halide perovskite LEDs. Nature 2021, 591, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Q.; Jin, Z.; Zhang, X.; Lei, J.; Bin, H.; Zhang, Z.G.; Li, Y.; Liu, S. Polymer doping for high-efficiency perovskite solar cells with improved moisture stability. Adv. Energy Mater. 2018, 8, 1701757. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite solar cells with 18.21% efficiency and area over 1 cm 2 fabricated by heterojunction engineering. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Duan, J.; Yang, X.; Tang, Q. Divalent Hard Lewis Acids Doped CsPbBr3 Films for 9.63%-Efficiency and Ultra-Stable All-Inorganic Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 6877–6882. [Google Scholar] [CrossRef]
- Girish, K.H. Advances in Surface passivation of perovskites using Organic halide salts for efficient and stable solar cells. Surf. Interfaces 2021, 26, 101420. [Google Scholar]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S. Hysteresis-free perovskite solar cells made of potassium-doped organometal halide perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Ma, Y.H.; Zhang, H.Y.; Zhang, Y.W.; Hu, R.Y.; Jiang, M.; Zhang, R.; Lv, H.; Tian, J.J.; Chu, L.; Zhang, J.; et al. Enhancing the Performance of Inverted Perovskite Solar Cells via Grain Boundary Passivation with Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 3044–3052. [Google Scholar] [CrossRef]
- Wei, J.; Li, H.; Zhao, Y.; Zhou, W.; Fu, R.; Wang, Y.L.; Yu, D.; Zhao, Q. Suppressed hysteresis and improved stability in perovskite solar cells with conductive organic network. Nano Energy 2016, 26, 139–147. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Hadadian, M.; Correa-Baena, J.P.; Goharshadi, E.K.; Ummadisingu, A.; Seo, J.Y.; Luo, J.; Gholipour, S.; Zakeeruddin, S.M.; Saliba, M.; Abate, A. Enhancing efficiency of perovskite solar cells via N-doped graphene: Crystal modification and surface passivation. Adv. Mater. 2016, 28, 8681–8686. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Zhao, S.; Zhang, H.; Li, H.; Zhuang, J.; Liu, X.; Li, H.; Wang, H. Defect passivation grain boundaries using 3-aminopropyltrimethoxysilane for highly efficient and stable perovskite solar cells. Sol. Energy 2021, 224, 472–479. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Li, M.; Shang, X.; Lei, K.; Zhang, B.; Chen, C.; Zheng, S.; Song, H.; Chen, J. Trap State Passivation by Rational Ligand Molecule Engineering toward Efficient and Stable Perovskite Solar Cells Exceeding 23% Efficiency. Adv. Energy Mater. 2021, 11, 2100529. [Google Scholar] [CrossRef]
- Hou, X.; Huang, S.; Ou-Yang, W.; Pan, L.; Sun, Z.; Chen, X. Constructing efficient and stable perovskite solar cells via interconnecting perovskite grains. ACS Appl. Mater. Interfaces 2017, 9, 35200–35208. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase–rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Boubaker, K. A physical explanation to the controversial Urbach tailing universality. Eur. Phys. J. Plus 2011, 126, 1–4. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem. Photobiol. 2012, 88, 257–264. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, Y.; Paek, S.; Gao, X.-X.; Li, X.; Nazeeruddin, M.K. Enhanced stability of α-phase FAPbI3 perovskite solar cells by insertion of 2D (PEA)2PbI4 nanosheets. J. Mater. Chem. A 2020, 8, 8058–8064. [Google Scholar] [CrossRef]
- Xiong, S.; Hou, Z.; Zou, S.; Lu, X.; Yang, J.; Hao, T.; Zhou, Z.; Xu, J.; Zeng, Y.; Xiao, W. Direct Observation on p-to n-Type Transformation of Perovskite Surface Region during Defect Passivation Driving High Photovoltaic Efficiency. Joule 2021, 5, 467–480. [Google Scholar] [CrossRef]
- Zhou, W.; Li, D.; Xiao, Z.; Wen, Z.; Zhang, M.; Hu, W.; Wu, X.; Wang, M.; Zhang, W.H.; Lu, Y. Zwitterion coordination induced highly orientational order of CH3NH3PbI3 perovskite film delivers a high open circuit voltage exceeding 1.2 V. Adv. Funct. Mater. 2019, 29, 1901026. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [Green Version]
- Xiang, W.; Chen, Q.; Wang, Y.; Liu, M.; Huang, F.; Bu, T.; Wang, T.; Cheng, Y.B.; Gong, X.; Zhong, J. Improved air stability of perovskite hybrid solar cells via blending poly (dimethylsiloxane)–urea copolymers. J. Mater. Chem. A 2017, 5, 5486–5494. [Google Scholar] [CrossRef]
- Chen, C.; Liu, D.; Zhang, B.; Bi, W.; Li, H.; Jin, J.; Chen, X.; Xu, L.; Song, H.; Dai, Q. Carrier interfacial engineering by bismuth modification for efficient and thermoresistant perovskite solar cells. Adv. Energy Mater. 2018, 8, 1703659. [Google Scholar] [CrossRef]
- Chen, C.; Liu, D.; Wu, Y.; Bi, W.; Sun, X.; Chen, X.; Liu, W.; Xu, L.; Song, H.; Dai, Q. Dual interfacial modifications by conjugated small-molecules and lanthanides doping for full functional perovskite solar cells. Nano Energy 2018, 53, 849–862. [Google Scholar] [CrossRef]






| Device | VTFL (V) | Ndefects (cm−3) | ||
|---|---|---|---|---|
| Hole-Only | Electron-Only | Hole-Only | Electron-Only | |
| control | 0.724 | 0.266 | 2.8 × 1016 | 1.05 × 1016 |
| with 0.05 wt% TCM | 0.564 | 0.138 | 2.2 × 1016 | 5.42 × 1015 |
| TCM (wt%) | Scan Direction | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Rs (Ω cm−2) |
|---|---|---|---|---|---|---|
| 0 | forward | 1.070 | 23.00 ± 0.62 | 76.46 ± 1.32 | 18.81 ± 0.31 | 55.98 |
| reverse | 1.067 | 22.63 ± 0.86 | 78.14 ± 1.43 | 18.87 ± 0.53 | 50.28 | |
| 0.01 | forward | 1.080 | 22.77 ± 0.57 | 77.81 ± 0.65 | 19.13 ± 0.52 | 43.75 |
| reverse | 1.073 | 22.75 ± 0.14 | 78.13 ± 0.43 | 19.07 ± 0.15 | 37.66 | |
| 0.05 | forward | 1.090 | 23.42 ± 0.12 | 78.32 ± 1.31 | 19.99 ± 0.45 | 32.97 |
| reverse | 1.085 | 22.69 ± 0.07 | 81.81 ± 1.32 | 20.15 ± 0.13 | 29.57 | |
| 0.1 | forward | 1.090 | 21.73 ± 0.65 | 77.59 ± 1.97 | 18.37 ± 0.58 | 50.32 |
| reverse | 1.091 | 21.20 ± 0.52 | 79.53 ± 0.64 | 18.39 ± 0.42 | 45.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Q.; Zhang, L.; Xu, Y.; Yuan, C.; Qi, W.; Fu, S.; Ma, Y.; Zeng, W.; Xia, R.; Min, Y. Defect Passivation Using Trichloromelamine for Highly Efficient and Stable Perovskite Solar Cells. Polymers 2022, 14, 398. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14030398
Niu Q, Zhang L, Xu Y, Yuan C, Qi W, Fu S, Ma Y, Zeng W, Xia R, Min Y. Defect Passivation Using Trichloromelamine for Highly Efficient and Stable Perovskite Solar Cells. Polymers. 2022; 14(3):398. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14030398
Chicago/Turabian StyleNiu, Qiaoli, Ling Zhang, Yao Xu, Chaochao Yuan, Weijie Qi, Shuai Fu, Yuhui Ma, Wenjin Zeng, Ruidong Xia, and Yonggang Min. 2022. "Defect Passivation Using Trichloromelamine for Highly Efficient and Stable Perovskite Solar Cells" Polymers 14, no. 3: 398. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14030398