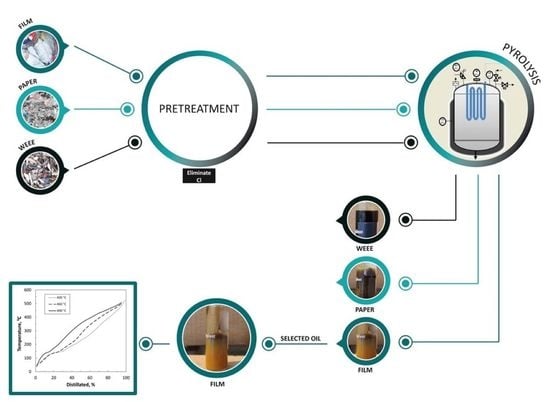
3.1.1. Properties of Waste Samples
Table 2 shows the material composition of the collected waste samples, both in the “raw” condition (as received) and after pre-treatment. Bulk density of samples is also included in
Table 2, together with two calculated ratios that allow quantifying the effectiveness of the pre-treatment techniques (see Equations (2) and (3)): “total material recovery” (TMR) and “recovery of material suitable for pyrolysis” (RMSP). While TMR refers to the amount of material obtained after pre-treatment (separation of unwanted materials), the term RMSP refers to the concentration of plastics in the recovered fraction that is appropriate for the pyrolysis process in order to obtain high liquid yields. In this research, polyolefins and styrenics were considered as the most suitable plastics for such an objective.
The raw Film sample is the sample that showed the highest plastic content, mainly composed of polyolefins (75 wt.%). In addition, its high content of PVC, PET and inorganic matter, the last formed by aluminium cans that were trapped inside the PE bags, was remarkable. In the flotation process, most of the polyolefins, whose density is less than 1.0 g cm
−3, floated to the surface while other polymers such as PVC and PET, and inorganic materials, whose densities are greater, sank to the bottom. Hence, the content of such undesirable components was significantly reduced during the pre-treatment. So, flotation was enabled to recover the 93.0 wt.% of the MSP, mainly formed by polyolefins (increase from 75.0 to 93.1 wt.%) with a high TMR (78.5 wt.%). It is reported that other authors employing flotation methods to separate plastics were used wetting agents in the process. Pongstabodee et al. used 30%
w/v calcium chloride solution to separate PP and HDPE from a mixed post-consumer plastic waste (PET, PVC, PS and ABS) [
31] and Guo et al. employed a solution with 70 mg L
−1 of sodium dodecyl sulphate to separate PS from a mixture of PET, PVC and PC from light packaging waste [
32]. However, in this case, the employment of wetting agents was not necessary to obtain high TMR and RMSP rates.
The raw Paper sample presented an important content of cellulosic materials (31.5 wt.%), principally paper and paperboard, which was expected because of the origin of the sample. Nevertheless, polyolefins constituted again the main fraction (36.1 wt.%). Moreover, it is important to highlight the high content of inorganic materials (13.4 wt.%) as well as the non-desired plastics, PVC (2.8 wt.%) and PET (5.3 wt.%) in the raw sample. In this case, the optical separation equipment achieved the removal of cellulosic material, reducing its content up to 18.3 wt.%. This resulted in a lower concentration of such oxygenated polymers, which might also improve the quality of the oil. It is also remarkable the strong reduction of inorganics (from 13.4 to 2.5 wt.%) and to a lesser extent that of PVC (from 2.8 to 1.5 wt.%) and PET (from 5.3 to 2.7 wt.%). However, compared to the other treatments, this method showed the lowest percentage of RMSP (43.7 wt.%). The separation difficulty of this sample lay in the fact that the paper was very intermingled with plastic and other materials and, in spite of the previous sample deagglomeration, the optical separation equipment could not properly identify and separate the desired polymers. In this case, the incorporation of a previous wet stage with some agent could have resulted in a better separation of polyolefins and paper. In fact, the dissolution of adhesive resins of polyolefins with the aim of separating polyolefins from post-consumer recycled paper was previously reported [
33].
The WEEE sample contained plastics of diverse nature, as can be observed from the high percentages of “other thermoplastics” (23.4 wt.%), which includes many different materials, and styrenics (39.2 wt.%), formed by ABS and PS. Additionally noteworthy was the high percentage of PVC, which in this case corresponded to electric wires. After passing through the densimetric table, the electrical wires were strongly reduced (from 16.3 to 4.8 wt.%) allowing to obtain 83.7 wt.% of RMSP and 67.2 wt.% of TMR. Hiosta et al. also applied this technique to separate electric wires from WEEE [
34]. Dodbiba et al. used the densimetric table to separate PP from PET/PVC fraction and concluded that the densimetric table was effective when the density difference between particles was at least 450 kg m
−3 [
35].
Concerning bulk densities (after being crushed to dp < 8 mm)
Table 2 shows that WEEE samples presented the highest values, whereas Film and Paper ones had extremely low densities. That means that Film and Paper samples could present more difficulties when stored, transported or fed to the reactor. The value of the bulk densities of the three samples decreased with the pre-treatments, mainly due to the removal of inorganics. In the WEEE sample, the difference was greater, probably owing to the decrease in the number of wires. In view of
Table 2, it can be said that, in general, the pre-treatment techniques employed have proved to be effective for concentrating the plastics, specifically the polyolefins, and reducing PVC, metal and other inorganic materials.
The ultimate and proximate analyses of both raw and pre-treated samples are presented in
Table 3 and
Table 4, respectively.
Table 3 also includes the HHV of the samples. As far as the ultimate analysis is concerned, the percentages of C, H and N corresponded adequately to the composition shown in
Table 2. The two samples with the highest plastic content (film and WEEE) showed the highest values of carbon while the sample with high paper content showed the typical carbon values of cellulosic materials. Concerning the H/C ratio, this was in accordance with the nature of the predominant polymers they contain, being the highest for polyolefin-rich samples (Film) and the lowest for styrene plastic-rich samples (WEEE) [
18,
22]. With regard to nitrogen, the high percentage of this element in the WEEE sample must be noted, which is directly related to its high content of nitrogenous polymers such as ABS, PUR or PA. Finally, it is worth noting the high percentage of chlorine in the Film and WEEE samples, which must mostly come from the PVC they contain. The WEEE sample has a much higher PVC content than the Film sample, and yet both have a chlorine content of around 4 wt.%. The explanation is that, as mentioned above, the PVC counted in the WEEE sample includes electric wires, i.e., it is not only PVC but also copper. After pre-treatment, an increase in C and H is generally appreciated due to the elimination of inorganic materials [
36], together with a noticeable decrease in chlorine, related to PVC elimination. This is a very important result in terms of producing pyrolysis oils with low chlorine content. At last, the Film sample showed the highest HHV as a consequence of its high polyolefinic content [
37], followed by the WEEE sample and the Paper sample, respectively. In all cases, the pre-treatment techniques caused an increase in HHV, as expected from the elimination of inorganic and low-HHV materials.
Regarding the proximate analysis, all samples were mainly composed of volatile matter, as expected in this type of plastic and paper-rich waste. This is a desirable property because it is from this volatile matter that the pyrolysis oils are formed. Otherwise, it can be seen that the paper samples contained higher moisture and fixed carbon than the rest, as expected from a sample rich in cellulosic material. In addition, the WEEE (raw) sample showed a significant amount of ash, probably coming from the PVC wires and inorganic fillers that may be contained in the plastics of this waste. The ash content was significantly reduced with pre-treatment (also in the other two samples), which increased the amount of volatile matter in the waste, a circumstance that would possibly improve the yield of pyrolysis oils, as mentioned above.
Table 5 shows the metal content of the samples. When analysing this table, the uncertainty associated with the multi-stage analysis of these complex samples must be taken into account. It can be seen that the major metals were calcium, titanium, aluminium and, in the case of the WEEE sample, copper from electric wires. It was surprising that the highest amount of iron was present in the paper sample, as this is an unsuitable material for paper/cardboard waste collection, although it is used in paper and printing ink applications [
38]. If such iron came from steel, it is possible that there were no magnetic separators in the waste paper and paperboard sorting plant, and this iron ended up in the rejected fraction under study in this work. Regarding heavy metals (Ni, Pb, Cd, Cr, As, Cu, Co, Tl, Sb, Sn, Hg, Mn, Zn), zinc was the most present, with the exception of copper in the case of the WEEE sample. On this occasion, no clear effect of the pre-treatment could be established for the three samples, although the reduction of the amount of copper in the WEEE sample was evident, which was in agreement with results obtained in previous characterizations.
The TGA profiles of all the samples are illustrated in
Figure 2, where it can be seen that temperatures slightly higher than 500 °C were needed for the total conversion of the three samples. In view of these results and taking into account that an isotherm of 1 h would be used in the pyrolysis experiments, a lower temperature (460 °C) was selected for the initial experiments, in order to avoid gas formation. As far as the decomposition phenomena occurring in the different samples are concerned, different behaviour can be observed between them. The Film sample showed a decomposition that took place practically in a single step at temperatures close to 500 °C, which is usual in samples whose main content is polyolefins [
39]. After pre-treatment, it seemed that decomposition happened in a lower temperature range (narrower DTG peak), which is a consequence of the removal of polymers that can start to decompose at lower temperatures than polyolefins (styrenics, PVC, etc.).
The thermogravimetric profile of the Paper sample showed three main stages of decomposition. (1) The first one close to 100 °C, corresponding to moisture loss, (2) another one around 350 °C, which is related to the decomposition of the cellulosic materials, and the last one (3), at temperatures similar to those observed for the Film sample, corresponding to the cracking of the polyolefins [
39]. After pre-treatment, the third DTG peak was higher, as a consequence of the polyolefin concentration resulting from pre-treatment.
Finally, the WEEE sample showed the classical decomposition phenomenon of PVC at 300 °C and the subsequent decomposition of the rest of the plastics, in this case in a wider temperature range than in the case of Film sample, due to the early decomposition of styrene plastics, compared to polyolefins [
20]. In fact, in the pre-treated sample, a decoupling at the peak of the main decomposition can be seen, due to the higher percentage of styrenics compared to the raw sample. A smaller peak size can also be observed at 300 °C, due to the lower PVC content.
3.1.2. Pyrolysis Process
The yields obtained in the pyrolysis of the three samples, raw and pre-treated, at 460 °C, are shown in
Table 6. Regarding the film-rich samples (raw and pre-treated), it can be seen that pyrolysis oils were the main product, followed by gas and solid. These results are directly attributed to the high polyolefin content in the initial sample (
Table 2). In particular, the liquid yield of the pre-treated film sample reached 70.6 wt.% owing to the 93.0% of RMSP present in the feedstock. These results are in accordance with those obtained in previous papers. Lopez et al. obtained 65 wt.% of liquid yield at 500 °C using a sample that contained 92.3 wt.% of plastic [
5]. Yan et al. reported the pyrolysis of PP and LDPE waste at 460 °C, reaching the 65.4 wt.% and 77.1 wt.% liquid yields, respectively [
40]. Regarding the effect of pre-treatment, the increase in the yield of liquids can be related to the decrease in the yield of solids. Such a decrease in solid yield can be explained by the elimination of inorganic compounds and polymers that have a tendency to carbonize (PVC, PET, cellulose) during the pre-treatment.
In the case of the Paper sample, the main fraction was the solid one, followed by liquids and gases. Such performance in solids can be explained by the high amount of inorganics contained in this sample (13.4 wt.%), together with a large amount of polymers with a tendency to carbonize, mainly cellulosic materials (31.5 wt.%). It is remarkable that this sample produced an aqueous liquid phase. This is explained by the presence of cellulosic-based materials rich in -OH and =O groups [
5,
20]. In the case of this sample, pre-treatment reduced the total solid yield by half and increased more than twice the organic liquid yield (from 17.8 to 42.5 wt.%). This fact might be explained by the significant effect of the pre-treatment on the reduction of (1) inorganic content (13.4 to 2.5 wt.%), and (2) char precursor materials, PET (5.3 to 2.7 wt.%) and especially cellulose (from 31.5 to 18.3 wt.%). However, the aqueous liquid phase could not be completely removed by the pre-treatment.
Finally, the WEEE sample generated also liquids as the main product, followed by solids and gases. Concerning the high solid yield, this sample did not contain a lot of inorganic material as such (2.3 wt.%), but it is necessary to remember the aforementioned issue of electric wires; in fact, the ash content determined by proximate analysis was high (22.0 wt.%,
Table 4). Furthermore, the group constituted by “other thermoplastics” contained polymers with a tendency to carbonize and within “multimaterial” there were some inorganic elements coming from circuit printed boards. After pre-treatment, the higher liquid yield was observed and, at the same time, the solid yield decreased, as a consequence of the removal of PVC, inorganics and multimaterial. The liquid yield obtained from these two WEEE samples was similar to those obtained by Caballero et al. when investigating the pyrolysis of WEEE plastics at 500 °C. They found that landline waste (phones) generated a 58 wt.% liquid yield while mobile phones a 54 wt.% [
22]. Higher values (around 70 wt.%) were obtained by Hall et al. during pyrolysis of mixed WEEE in a fixed bed reactor at 600 °C [
41].
To summarize, it can be said that for the three different waste samples, the pre-treatment led to higher liquid yields and lower solid yields as compared to the pyrolysis of raw samples, while gas yields remained almost constant. This is the evidence that the pre-treatments produced the desired effect, which is the promotion of pyrolysis oils through the elimination of undesired materials of the original samples.
3.1.3. Pyrolysis Oils
Liquid products had a different appearance depending on the composition of the pyrolyzed waste sample. The liquids obtained from Film waste samples resulted in a waxy-like product instead of liquid oil, which can be ascribed to the high H/C ratio of the waste samples (see
Table 3), principally explained by their great PE content [
42,
43]. In fact, Kiran et al. and Sharudin et al. experimented with operational blockage problems in pipelines and condenser tubes with waxes formation when pyrolyzed samples richer in PE [
44,
45]. Nevertheless, these waxes obtained from the polyolefins pyrolysis can serve as feedstock for FCC units of petroleum refineries [
46,
47]. Paper samples presented two differenced phases (organic and aqueous) in the liquid as was explained in
Section 3.1.2. The organic phase of the pre-treated paper oil presented a more waxy-like appearance than the non-pre-treated one according to the promotion of polyolefins with the pre-treatment (see
Table 2). By contrast, the pyrolysis of WEEE samples, with an aromatic/naphthenic nature, result in dark-brown coloured oils, which resemble petroleum fractions [
5,
18,
22].
In order to evaluate the quality of the organic liquid products, several of their properties, such as higher heating value (HHV), halogen and metal content, and composition, were determined. First, the most limiting properties, i.e., HHV, halogen content and metal content, were analysed. This information is presented in
Table 7 and
Table 8.
Table 7 shows the HHV and the halogen content of these liquid products. The HHV of the pyrolysis oils was high (40–43 MJ kg
−1) and close to those of liquid fossil fuels (diesel 45 MJ kg
−1 and heavy fuel oil 42–43 MJ kg
−1 [
43]), with the exception of Paper samples (37–39 MJ kg
−1). This is an important result, as it provides the possibility of using these oils as alternative fuels. Again, the pre-treatment improved the calorific properties of the pyrolysis oils, increasing the HHV in all cases due to the reduction of impurities and PET [
36], and the concentration of MSP.
On the other hand, it is important to consider the halogen content, since they have an important and negative impact on the direct application of pyrolysis oils as fuels [
20,
48]. In this work, fluorine, chlorine and bromine were measured. It is clear from
Table 7 that the main halogen element in the pyrolysis oils was chlorine. The fluorine and bromine values were very low, with the exception of the bromine content of the liquids from the WEEE samples, probably due to the presence of brominated flame retardants as part of the additives in the plastic materials of this waste. Liquids generated from raw waste samples presented more chlorine content, directly related to the presence of PVC, as was concluded by several authors [
20,
49,
50]. Although all pre-treatments achieved a reduction in the halogen content, pyrolysis liquids still showed relatively high chlorine content. PT-Film sample registered the lowest chlorine content (160 ppm). This value, although low, is higher than the value established for use in existing petrochemical plants (3–10 ppm), as stated by some authors [
51,
52,
53] However, these pyrolysis oils could probably be blended with other refinery streams before usage and most likely could be used as alternative fuels in cement kilns, where the required chlorine concentration is not usually so low. In the conditions of this work, about 10–30 wt.% of the chlorine content present in the waste samples was transferred to the liquid product. These transfer ratios can be reduced in several ways: using solid catalysts or adsorbents [
20,
49,
54] or by the application of stepwise pyrolysis (two-stage pyrolysis) [
55] but this was out of the scope of this paper.
Table 8 shows the metal content of the pyrolysis oils. In this case, two issues need to be considered: the heavy metal content, which could lead to environmental problems, and the presence of metals that could act as poisons in catalysts used in petrochemical processes. With regard to heavy metals (in bold in the table), zinc, antimony, lead, nickel, manganese and chromium were detected, all in concentrations below 8 ppm in the oils from the pre-treated samples. This means that all these oils were free of heavy metals such as cadmium, copper, arsenic, cobalt, thallium, tin and mercury, or at least the concentration of these metals was below 1 ppm. Among the metals that can cause problems in catalysts, the presence of silicon was particularly noticeable in the oils from the WEEE samples. For this reason, it is important to take it into account when processing oil in the refinery, since requirements are usually established to avoid its presence and prevent damage to the catalysts. The limits of the metals will depend on each refinery, the processing unit in which the oil is included and the degree of dissolution that the oil presents along with the conventional feed used.
As the liquid fractions coming from the pre-treated samples showed better quality, the characterization by the GC/MS was carried out only in these oils.
Figure 3 shows the compounds identified by this method grouped according to their nature in paraffinic, naphthenic, olefinic and aromatic compounds. Only those compounds with areas > 1% and an identification quality degree > 90% were included in such groups. The oil from the pre-treated Film sample was composed mainly of paraffins (59.9% area) and olefins (30.2% area), due to the high content of PE presented in the original sample (see
Table 2). It was proven by other authors that during the degradation of PE, free radical fragments are formed and react with hydrogen chains, giving rise to alkanes and alkenes [
5,
56]. According to Das et al., olefins are the precursors of many industrial organic chemicals such as vinyl acetate, acetaldehyde and vinyl chloride, therefore, the concentration of olefins in the pyrolytic oil could be used in numerous industrial applications [
57].
On the other hand, pre-treated WEEE sample oil consisted of more than 97% area of aromatic compounds, with small quantities of naphthenes (2.4% area). The high quantity of aromatics is attributed to the great styrene content and the low content of polyolefins in the original sample (see
Table 2). In previous investigations, 80% of aromatic hydrocarbons were obtained in the pyrolysis of PS [
58]. Since a high concentration of aromatics is desired for gasoline production [
10], this could be the most appropriate application for PT-WEEE oil provided the chlorine content is reduced. The major compounds in the PT-Paper sample oil included paraffins (45.8% area), naphthenes (16.9% area) and aromatics (34.5% area). This wide distribution is related to the composition of the sample. As was previously mentioned, polyolefins generate paraffinic and olefinic compounds while styrenics favour aromatic content in the liquid oils. Moreover, other fractions such as PET could also favour the formation of the former compounds [
15].