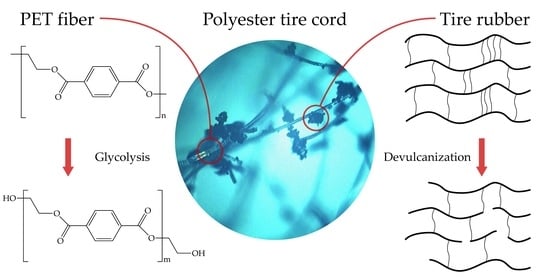
Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycolysis
2.2.1. Synthesis of Glycolytic Agents
2.2.2. Heterogeneous Glycolysis
2.2.3. Novel Glycolysis
2.3. Cord Characterization
2.4. Characterization of Glycolysates
2.4.1. PET Conversion
2.4.2. Gel Permeation Chromatography (GPC)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Characterization of Reclaimed Rubber
2.5.1. Degree of Devulcanization
2.5.2. Horikx Plot
2.5.3. Swelling Characterization
3. Results and Discussion
3.1. Textile Cord Characterization
3.2. Obtained Glycolysis Agents Properties
3.3. Textile Cord Glycolysis Investigation
3.4. Devulcanization Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watt, E.; Picard, M.; Maldonado, B.; Abdelwahab, M.A.; Mielewski, D.F.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Ocean plastics: Environmental implications and potential routes for mitigation—A perspective. RSC Adv. 2021, 11, 21447–21462. [Google Scholar] [CrossRef]
- Dabic-Miletic, S.; Simic, V.; Karagoz, S. End-of-life tire management: A critical review. Environ. Sci. Pollut. Res. 2021, 28, 68053–68070. [Google Scholar] [CrossRef]
- Moghaddamzadeh, S.; Rodrigue, D. The effect of polyester recycled tire fibers mixed with ground tire rubber on polyethylene composites. Part I: Morphological analysis. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 200–220. [Google Scholar] [CrossRef]
- Moghaddamzadeh, S.; Rodrigue, D. The effect of polyester recycled tire fibers mixed with ground tire rubber on polyethylene composites. Part II: Physico-mechanical. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 128–142. [Google Scholar] [CrossRef]
- Rahimi, R.S.; Nikbin, I.M.; Allahyari, H.; Habibi, T.S. Sustainable approach for recycling waste tire rubber and polyethylene terephthalate (PET) to produce green concrete with resistance against sulfuric acid attack. J. Clean. Prod. 2016, 126, 166–177. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Banthia, N. Durability performance of polymeric scrap tire fibers and its reinforced cement mortar. Mater. Struct. 2017, 50, 158. [Google Scholar] [CrossRef]
- Figueiredo, F.P.; Angelakopoulos, H. Effects of Recycled Steel and Polymer Fibres on Explosive Fire Spalling of Concrete. Fire Technol. 2019, 55, 1495–1516. [Google Scholar] [CrossRef] [Green Version]
- Manaf, A.F.A.; Shahidan, S.; Shamsuddin, S.-M.; Sharif, N.F.; Zuki, S.S.M.; Khalid, F.S.; Ali, N.; Ali, M.; Azmi, M. Efficiency of Polyethylene Terephthalate (PET) Waste Fiber in Concrete Material by Means of Ultrasonic Velocity Method. SSRG Int. J. Eng. Trends Technol. 2020, 68, 18–24. [Google Scholar] [CrossRef]
- Gordeeva, I.V.; Melnikov, D.A.; Gorbatova, V.N.; Reznichenko, D.S.; Naumova, Y.A. Investigation of modified bitumen binders via Fourier-transform infrared spectroscopy. Fine Chem. Technol. 2020, 15, 56–66. [Google Scholar] [CrossRef]
- Xu, X.; Leng, Z.; Lan, J.; Wang, W.; Yu, J.; Bai, Y.; Sreeram, A.; Hu, J. Sustainable Practice in Pavement Engineering through Value-Added Collective Recycling of Waste Plastic and Waste Tyre Rubber. Engineering 2020, 7, 857–867. [Google Scholar] [CrossRef]
- Narani, S.S.; Abbaspour, M.; Mir Mohammad Hosseini, S.M.; Aflaki, E.; Moghadas Nejad, F. Sustainable Reuse of Waste Tire Textile Fibers (WTTFs) as Reinforcement Materials for Expansive Soils: With a Special Focus on Landfill Liners/Covers. J. Clean. Prod. 2020, 247, 119151. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Katalagarianakis, A.; Huysman, S.; Toncheva, A.; Raquez, J.-M.; Duretek, I.; Holzer, C.; Cardon, L.; Bernaerts, K.V.; Van Hemelrijck, D.; et al. Effect of extrusion and fused filament fabrication processing parameters of recycled poly(ethylene terephthalate) on the crystallinity and mechanical properties. Addit. Manuf. 2022, 50, 102518. [Google Scholar] [CrossRef]
- Toncheva, A.; Brison, L.; Dubois, P.; Laoutid, F. Recycled Tire Rubber in Additive Manufacturing: Selective Laser Sintering for Polymer-Ground Rubber Composites. Appl. Sci. 2021, 11, 8778. [Google Scholar] [CrossRef]
- Laoutid, F.; Lafqir, S.; Toncheva, A.; Dubois, P. Valorization of Recycled Tire Rubber for 3D Printing of ABS- and TPO-Based Composites. Materials 2021, 14, 5889. [Google Scholar] [CrossRef]
- Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone towardsustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Barnard, E.; Jonathan, J.; Arias, R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gerval’d, A.Y.; Toms, R.V. Obtaining oligoesters by directed glycolytic destruction of polyethylene terephthalate waste. Plast. Massy 2020, 1, 51–53. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V. Study of polyethylene terephthalate glycolysis with a mixture of bis(2-hydroxyethyl) terephthalate and its oligomers. Plast. Massy 2021, 1, 50–52. [Google Scholar] [CrossRef]
- Simsek, B. Bis-hydroxyethyl terephthalate cementitious composites properties: A comparative study including hydrogen bonding mechanism with the dioctyl terephthalate. Constr. Build Mater. 2020, 258, 119691. [Google Scholar] [CrossRef]
- Korolchuk, A.A.; Zhavoronok, E.S.; Legonkova, O.A.; Kedik, S.A. Effect of polyethylene glycol mixtures as ointment base on the physicochemical properties of Lavsan atraumatic wound dressings. Fine Chem. Technol. 2019, 14, 71–78. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gerval’d, A.Y.; Toms, R.V.; Balashov, M.S. Natural Latex Deproteinization Issues. Kauchuk i Rezina 2020, 79, 310–316. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gerval’d, A.Y. Elastomeric Compositions in Wound Dressings. Kauchuk i Rezina 2021, 80, 150–154. [Google Scholar] [CrossRef]
- Macsiniuc, A.; Rochette, A.; Rodrigue, D. Understanding the Regeneration of EPDM Rubber Crumbs from Used Tyres. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 51–81. [Google Scholar] [CrossRef]
- Gursel, A.; Akca, E.; Sen, N. A Review on Devulcanization of Waste Tire Rubber. Period. Eng. Nat. Sci. 2018, 6, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Seghar, S.; Asarob, L.; Rolland-Monneta, M.; Ait Hocine, N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [Green Version]
- Stoski, A.; Viante, M.F.; Nunes, C.S.; Muniz, E.C.; Felsner, M.L.; Almedia, C.A.P. Oligomer production through glycolysis of poly(ethylene terephthalate): Effects of temperature and water content on reaction extent. Polym. Int. 2016, 65, 1024–1030. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Kovaleva, L.A.; Ovsyannikov, N.Y.; Zuev, A.A. Change of electrical characteristics of rubber in the process of “swelling–deswelling”. Fine Chem. Technol. 2020, 15, 56–66. [Google Scholar] [CrossRef]
- Tarasova, N.; Zanin, A.; Krivoborodov, E.; Toropygin, I.; Pascal, E.; Mezhuev, Y. The New Approach to the Preparation of Polyacrylamide-Based Hydrogels: Initiation of Polymerization of Acrylamide with 1,3-Dimethylimidazolium (Phosphonooxy-)Oligosulphanide under Drying Aqueous Solutions. Polymers 2021, 13, 1806. [Google Scholar] [CrossRef] [PubMed]
- Ziyu, C.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]






| Sample | Gl-1 | Gl-2 | Gl-3 |
|---|---|---|---|
| Glycolysis method | Heterogeneous glycolysis | Novel glycolysis | |
| Glycolytic agents | EG | DEG | OET, BHET, EG |
| Temperature, °C | 190 | 220 | 270, 250, 220, 190 |
| Molar ratio of agent (agent units) to PET units | 9:1 | 9:1 | 3:1, 3:1, 3:1 |
| Sample | PET Conversion, % | Mn | Mw | PDI |
|---|---|---|---|---|
| Gl-1 | 5 | 101 | 146 | 1.44 |
| Gl-2 | 12 | 120 | 148 | 1.23 |
| Gl-3 | 100 | 323 | 445 | 1.38 |
| Sample | Soluble Fraction, % | Density, g/mol | Crosslink Density, ×10−5 mol/cm3 | Apparent Degree of Swelling, % | Apparent Swelling Rate Constant, min−1 |
|---|---|---|---|---|---|
| RBD | 0 | 1.056 | 3.96 | 108.7 | 0.0483 |
| Gl-1 | 3.28 | 1.042 | 3.08 | 127.9 | 0.0645 |
| Gl-2 | 4.71 | 1.037 | 2.83 | 134.0 | 0.0803 |
| Gl-3 | 5.99 | 1.015 | 2.14 | 167.4 | 0.0902 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization. Polymers 2022, 14, 684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14040684
Kirshanov K, Toms R, Melnikov P, Gervald A. Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization. Polymers. 2022; 14(4):684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14040684
Chicago/Turabian StyleKirshanov, Kirill, Roman Toms, Pavel Melnikov, and Alexander Gervald. 2022. "Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization" Polymers 14, no. 4: 684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14040684