Heavy Metal Ions Removal from Aqueous Solutions by Treated Ajwa Date Pits: Kinetic, Isotherm, and Thermodynamic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Biosorbent Preparation
2.4. Adsorption and Desorption Studies
2.5. Thermodynamic Studies of Cu(II) Ions Adsorption
3. Results and Discussion
3.1. Characterization
3.1.1. Surface and Pore Size Analysis of Adsorbents
3.1.2. FTIR
3.2. Adsorption Properties
3.2.1. Effect of pH of the Solution
3.2.2. Effect of Initial Concentration and Temperature
3.2.3. Effects of Contact Time
3.2.4. Adsorption Modeling
Equilibrium Isotherm
3.3. Adsorption Kinetics
3.4. Adsorption Thermodynamics
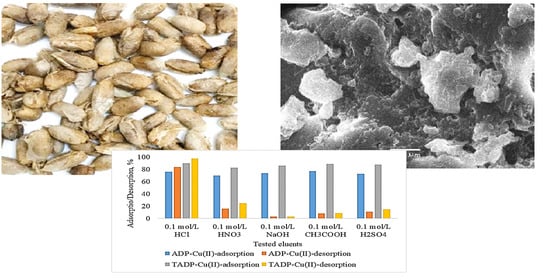
3.5. Elution and Regeneration Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.-M.; Fu, R.-B.; Tong, Y.-H.; Shen, D.-L.; Guo, X.-P. The potential environmental risk implications of heavy metals based on their geochemical and mineralogical characteristics in the size-segregated zinc smelting slags. J. Clean. Prod. 2021, 315, 128199. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Liu, H.; Khan, M.R.; Zhao, Z.; He, G.; Luo, A.; Zhang, J.; Deng, R.; He, Q. Label-free DNAzyme assays for dually amplified and one-pot detection of lead pollution. J. Hazard. Mater. 2021, 406, 124790. [Google Scholar] [CrossRef]
- Khan, M.R.; Ahmad, N.; Ouladsmane, M.; Azam, M. Heavy Metals in Acrylic Color Paints Intended for the School Children Use: A Potential Threat to the Children of Early Age. Molecules 2021, 26, 2375. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 3, 1–7. [Google Scholar] [CrossRef]
- SenthilKumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Sivanesan, S. Removal of copper (II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 2011, 266, 63–71. [Google Scholar] [CrossRef]
- Zhu, C.S.; Wang, L.P.; Chen, W.B. Removal of Cu (II) from aqueous solution by agricultural by-product: Peanut hull. J. Hazard. Mater. 2009, 168, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Zhou, H.; Ye, T.; Huang, Z.; Liu, R.; Kuang, Y. Highly efficient removal of Cu (II) from aqueous solution by using graphene oxide. Water Air Soil Pollut. 2013, 224, 1–8. [Google Scholar] [CrossRef]
- Singha, B.; Das, S.K. Adsorptive removal of Cu (II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surf. B Biointerfaces 2013, 107, 97–106. [Google Scholar] [CrossRef]
- Edding, M.; Tala, F. Copper transfer and influence on a marine food chain. Bull. Environ. Contam. Toxicol. 1996, 57, 617–624. [Google Scholar] [CrossRef]
- Stenhammar, L. Diarrhoea following contamination of drinking water with copper. Eur. J. Med. Res. 1999, 4, 217–218. [Google Scholar]
- Ross, A. Vomiting and Diarrhoea due to Copper in Stewed Apples. Lancet 1955, 87–88. [Google Scholar] [CrossRef]
- Bergeron, C.; Petrunka, C.; Weyer, L. Copper/zinc superoxide dismutase expression in the human central nervous system. Correlation with selective neuronal vulnerability. Am. J. Pathol. 1996, 148, 273. [Google Scholar] [PubMed]
- Araya, M.; Peña, C.; Pizarro, F.; Olivares, M. Gastric response to acute copper exposure. Sci. Total Environ. 2003, 303, 253–257. [Google Scholar] [CrossRef]
- Acrylamide, O. National Primary Drinking Water Regulations. Kidney 2009, 2, 1–7. [Google Scholar]
- Li, Y.; Trush, M.A.; Yager, J.D. DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol. Carcinogenesis 1994, 15, 1421–1427. [Google Scholar] [CrossRef]
- Starkebaum, G.; Harlan, J.M. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J. Clin. Investig. 1986, 77, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renfew, M.M. NIOSH Pocket Guide to Chemical Hazards; ACS Publications; US Department of Health and Human Services—National Institute for Occupational Safety and Health: Washington, DC, USA, 1991. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Un, U.T.; Ocal, S.E. Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. Int. J. Environ. Sci. Dev. 2015, 6, 425. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, L.; Xu, Z.; Sun, Q. Separation and Recovery of Heavy Metals from Waste Water Using Synergistic Solvent Extraction; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- BrbootI, M.M.; AbiD, B.A.; Al-ShuwaikI, N.M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J 2011, 29, 595–612. [Google Scholar]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Sunil, K.; Karunakaran, G.; Yadav, S.; Padaki, M.; Zadorozhnyy, V.; Pai, R.K. Al-Ti2O6 a mixed metal oxide based composite membrane: A unique membrane for removal of heavy metals. Chem. Eng. J. 2018, 348, 678–684. [Google Scholar] [CrossRef]
- Wu, S.; Xu, Y.; Sun, J.; Cao, Z.; Zhou, J.; Pan, Y.; Qian, G. Inhibiting evaporation of heavy metal by controlling its chemical speciation in MSWI fly ash. Fuel 2015, 158, 764–769. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S.; Dorris, K.L. The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J. Hazard. Mater. 2000, 80, 33–42. [Google Scholar] [CrossRef]
- Lu, S.; Gibb, S.W. Copper removal from wastewater using spent-grain as biosorbent. Bioresour. Technol. 2008, 99, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Gao, C.; Ma, J.; Ma, X.; Han, R. Adsorption of copper ions and methylene blue in a single and binary system on wheat straw. J. Chem. Eng. Data 2009, 54, 3229–3234. [Google Scholar] [CrossRef]
- Ofomaja, A.; Naidoo, E.; Modise, S. Removal of copper (II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J. Hazard. Mater. 2009, 168, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Larous, S.; Meniai, A. Removal of copper (II) from aqueous solution by agricultural by-products-sawdust. Energy Procedia 2012, 18, 915–923. [Google Scholar]
- Gündoğan, R.; Acemioğlu, B.; Alma, M.H. Copper (II) adsorption from aqueous solution by herbaceous peat. J. Colloid Interface Sci. 2004, 269, 303–309. [Google Scholar] [CrossRef]
- Aksu, Z.; İşoğlu, İ.A. Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Hanafiah, M.; ALOthman, Z.A.; Khan, M.R.; Surip, S. Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: Modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J. Chem. Eng. 2021, 38, 1–11. [Google Scholar] [CrossRef]
- Jawad, A.H.; Bardhan, M.; Islam, M.A.; Islam, M.A.; Syed-Hassan, S.S.A.; Surip, S.; ALOthman, Z.A.; Khan, M.R. Insights into the modeling, characterization and adsorption performance of mesoporous activated carbon from corn cob residue via microwave-assisted H3PO4 activation. Surf. Interfaces 2020, 21, 100688. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Wilson, L.D.; Syed-Hassan, S.S.A.; ALOthman, Z.A.; Khan, M.R. High surface area and mesoporous activated carbon from KOH-activated Dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Removal of Chromium (III) and Cadmium (II) Heavy Metal Ions from Aqueous Solutions Using Treated Date Seeds: An Eco-Friendly Method. Molecules 2021, 26, 3718. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Adekoya, A.E.; Idowu, S. Dates palm fruits: A review of their nutritional components, bioactivities and functional food applications. AIMS Agric. Food 2020, 5, 734–755. [Google Scholar] [CrossRef]
- Khan, M.A.; Otero, M.; Kazi, M.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Alothman, Z.A.; Sumbul, S. Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. J. Hazard. Mater. 2019, 365, 759–770. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.; Lee, S. Optimization of Cd (II), Cu (II) and Ni (II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A. Development of citric anhydride anchored mesoporous MOF through post synthesis modification to sequester potentially toxic lead (II) from water. Microporous Mesoporous Mater. 2018, 261, 198–206. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Khan, M.A.; Siddiqui, M.R.; Otero, M.; Jeon, B.-H.; Alothman, Z.A.; Hakami, A.A.H. Oxygenated functionalities enriched MWCNTs decorated with silica coated spinel ferrite–A nanocomposite for potentially rapid and efficient de-colorization of aquatic environment. J. Mol. Liq. 2020, 317, 113916. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E. Heteroatom-doped magnetic hydrochar to remove post-transition and transition metals from water: Synthesis, characterization, and adsorption studies. Chemosphere 2019, 218, 1089–1099. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly (vinyl alcohol) beads functionalized with poly (ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and equilibrium models of adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 33–69. [Google Scholar]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]









| Element | (keV) | Mass% | |||
|---|---|---|---|---|---|
| ADP | Error | TADP | Error | ||
| C K | 0.277 | 45.37 | 0.15 | 42.96 | 0.21 |
| N K | 0.392 | 25.60 | 0.68 | 21.18 | 0.45 |
| O K | 0.525 | 29.03 | 0.18 | 35.86 | 0.16 |
| Total | 100 | 100 | |||
| Isotherm | Temperature, K | |||
|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |
| TADP | ||||
| Langmuir qm (mg/g) KL (L/mg) R2 | 666.67 0.057915 0.9889 | 909.09 0.01449 0.9972 | 1111.11 0.01289 0.9986 | 1428.57 0.01107 0.9932 |
| Freundlich KF (mg/g) (L/mg)1/n n R2 | 19.1134 1.2449 0.9096 | 37.6320 1.2358 0.9253 | 53.6320 1.2858 0.9170 | 57.0176 1.4550 0.9250 |
| ADP | ||||
| Langmuir qm (mg/g) KL (L/mg) R2 | 348.0 0.03275 0.9853 | 625.0 0.07048 0.9901 | 769.2 0.01289 0.9932 | 1111.1 0.03904 0.9891 |
| Freundlich KF (mg/g) (L/mg)1/n n R2 | 18.52 1.0132 0.7192 | 23.43 1.2168 0.8586 | 23.69 1.6211 0.8914 | 48.56 1.7621 0.8979 |
| Kinetics Model | ADP | TADP |
|---|---|---|
| Cu(II) 20 mg/L | ||
| qe,exp (mg/g) | 136 | 178 |
| Pseudo-first-order | ||
| qe1,cal (mg/g) | 118.38 | 126.88 |
| K1(1/min) | 0.0097 | 0.0145 |
| R2 | 0.991 | 0.978 |
| Pseudo-second-order | ||
| qe2,cal (mg/g) | 145.25 | 189.07 |
| k2(g/mg-min) | 0.00005 | 0.00003 |
| R2 | 0.996 | 0.995 |
| Adsorbents | Concentration mg/L | ΔH° | ΔS° | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| (kJ/mol) | (J/mol-K) | 298 K | 308 K | 318 K | 328 K | ||
| ADP | 20 | 2608.34 | 86.29 | −156.34 | −566.44 | −951.43 | −1020.48 |
| 30 | 2122.10 | 69.92 | −165.79 | −341.35 | −708.64 | −916.92 | |
| 40 | 1810.70 | 60.54 | −419.21 | −479.90 | −562.61 | −821.86 | |
| 50 | 1762.03 | 59.16 | −64.62 | −92.97 | −423.11 | −634.68 | |
| TADP | 20 | 1017.01 | 41.52 | −2167.69 | −2537.27 | −2934.88 | −3064.55 |
| 30 | 704.95 | 28.99 | −1533.09 | −1904.93 | −2098.57 | −2178.40 | |
| 40 | 854.00 | 34.71 | −1786.02 | −2073.66 | −2380.42 | −2550.16 | |
| 50 | 896.01 | 30.83 | −257.90 | −409.23 | −899.72 | −1004.94 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Heavy Metal Ions Removal from Aqueous Solutions by Treated Ajwa Date Pits: Kinetic, Isotherm, and Thermodynamic Approach. Polymers 2022, 14, 914. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050914
Azam M, Wabaidur SM, Khan MR, Al-Resayes SI, Islam MS. Heavy Metal Ions Removal from Aqueous Solutions by Treated Ajwa Date Pits: Kinetic, Isotherm, and Thermodynamic Approach. Polymers. 2022; 14(5):914. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050914
Chicago/Turabian StyleAzam, Mohammad, Saikh Mohammad Wabaidur, Mohammad Rizwan Khan, Saud I. Al-Resayes, and Mohammad Shahidul Islam. 2022. "Heavy Metal Ions Removal from Aqueous Solutions by Treated Ajwa Date Pits: Kinetic, Isotherm, and Thermodynamic Approach" Polymers 14, no. 5: 914. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050914