Polylactide-Grafted Metal-Alginate Aerogels
Abstract
:1. Introduction
2. Materials and Methods
Synthesis of PLA-Grafted M-Alginate (g-M-Alginate) Aerogels
3. Results and Discussion
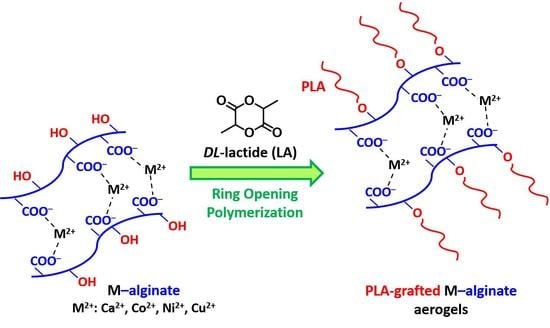
3.1. Preparation and Chemistry of PLA-Grafted M-Alginate (g-M-Alginate) Aerogels
3.2. Chemical Characterization of PLA-Grafted M-Alginate (g-M-Alginate) Aerogels
3.3. Selected Material Properties of PLA-Grafted M-Alginate (g-M-Alginate) Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click Synthesis of Monolithic Silicon Carbide Aerogels from Polyacrylonitrile-Coated 3D Silica Networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A Reconsideration on the Definition of the Term Aerogel Based on Current Drying Trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Kistler, S.S. The Relation between Heat Conductivity and Structure in Silica Aerogel. J. Phys. Chem. 1935, 39, 79–86. [Google Scholar] [CrossRef]
- Kistler, S.S.; Swann, S.; Appel, E.G. Aerogel Catalysts—Thoria: Preparation of Catalyst and Conversions of Organic Acids to Ketones. Ind. Eng. Chem. 1934, 26, 388–391. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science & Business Media, LLC: New York, NY, USA, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Raman, S.P.; Gurikov, P.; Smirnova, I. Hybrid Alginate Based Aerogels by Carbon Dioxide Induced Gelation: Novel Technique for Multiple Applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-Based Aerogels—Promising Biodegradable Carriers for Drug Delivery Systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-Based Aerogels as Drug Carriers. J. Non-Cryst. Solids 2009, 355, 2472–2479. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Renzo, F.D. Aerogel Materials from Marine Polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A Novel Approach to Alginate Aerogels: Carbon Dioxide Induced Gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Renzo, F.D.; Quignard, F. Nanostructure of Calcium Alginate Aerogels Obtained from Multistep Solvent Exchange Route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Gurikov, P.; Raptopoulos, G.; Chriti, D.; Papastergiou, M.; Kypritidou, Z.; Skounakis, V.; Argyraki, A. Strategies toward Catalytic Biopolymers: Incorporation of Tungsten in Alginate Aerogels. Polyhedron 2018, 154, 209–216. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Cheng, J.-B.; Wang, Y.-Z. Biomass-Derived Co@crystalline Carbon@carbon Aerogel Composite with Enhanced Thermal Stability and Strong Microwave Absorption Performance. J. Alloys Compd. 2018, 736, 71–79. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium−Alginate Gels with X-ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Trens, P.; Valentin, R.; Quignard, F. Cation Enhanced Hydrophilic Character of Textured Alginate Gel Beads. Colloids Surf. A Physicochem. Eng. Aspects 2007, 296, 230–237. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Raptopoulos, G.; Leontaridou, F.; Papastergiou, M.; Sakellari, A.; Karavoltsos, S. Evaluation of Polyurea-Crosslinked Alginate Aerogels for Seawater Decontamination. Gels 2021, 7, 27. [Google Scholar] [CrossRef]
- Pignolet, L.H.; Waldman, A.S.; Schechinger, L.; Govindarajoo, G.; Nowick, J.S.; Labuza, T. The Alginate Demonstration: Polymers, Food Science, and Ion Exchange. J. Chem. Educ. 1998, 75, 1430. [Google Scholar] [CrossRef]
- Mohite, D.P.; Larimore, Z.J.; Lu, H.; Mang, J.T.; Sotiriou-Leventis, C.; Leventis, N. Monolithic Hierarchical Fractal Assemblies of Silica Nanoparticles Cross-Linked with Polynorbornene via ROMP: A Structure–Property Correlation from Molecular to Bulk through Nano. Chem. Mater. 2012, 24, 3434–3448. [Google Scholar] [CrossRef]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically Strong Aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Vassilaras, P.; Fabrizio, E.F.; Dass, A. Polymer Nanoencapsulated Rare Earth Aerogels: Chemically Complex but Stoichiometrically Similar Core–Shell Superstructures with Skeletal Properties of Pure Compounds. J. Mater. Chem. 2007, 17, 1502–1508. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Mandal, C.; Donthula, S.; Far, H.M.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Transparent, Mechanically Strong, Thermally Insulating Cross-Linked Silica Aerogels for Energy-Efficient Windows. J. Sol-Gel Sci. Technol. 2019, 92, 84–100. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Chatzichristidi, M.; Raptopoulos, G.; Gurikov, P. Mechanically Strong Polyurea/Polyurethane-Cross-Linked Alginate Aerogels. ACS Appl. Polym. Mater. 2020, 2, 1974–1988. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Raptopoulos, G.; Gurikov, P. Polyurea-Crosslinked Biopolymer Aerogel Beads. RSC Adv. 2020, 10, 40843. [Google Scholar] [CrossRef]
- Raptopoulos, G.; Papastergiou, M.; Chriti, D.; Effraimopoulou, E.; Čendak, T.; Samartzis, N.; Mali, G.; Ioannides, T.; Gurikov, P.; Smirnova, I.; et al. Metal-Doped Carbons from Polyurea-Crosslinked Alginate Aerogel Beads. Mater. Adv. 2021, 2, 2684–2699. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Raptopoulos, G.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Fundamental Skeletal Nanostructure of Nanoporous Polymer-Cross-Linked Alginate Aerogels and Its Relevance to Environmental Remediation. ACS Appl. Nano Mater. 2021, 4, 10575–10583. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Ghimire, S.; Sala, M.R.; Chandrasekaran, S.; Raptopoulos, G.; Worsley, M.; Paraskevopoulou, P.; Leventis, N.; Sabri, F. Noninvasive Detection, Tracking, and Characterization of Aerogel Implants Using Diagnostic Ultrasound. Polymers 2022, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of Cellulose by Ring-Opening Polymerisation—A Review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef] [Green Version]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-Graft-Poly(L-Lactide) Copolymers for Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lönnberg, H.; Zhou, Q.; Brumer, H.; Teeri, T.T.; Malmström, E.; Hult, A. Grafting of Cellulose Fibers with Poly(ε-Caprolactone) and Poly(l-Lactic Acid) via Ring-Opening Polymerization. Biomacromolecules 2006, 7, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xu, Q.; Li, Y.; Mo, S.; Cai, S.; Liu, L. The Synthesis of Biodegradable Graft Copolymer Cellulose-Graft-Poly(L-Lactide) and the Study of Its Controlled Drug Release. Colloids Surf. B Biointerfaces 2008, 66, 26–33. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, S.; Bao, H.; Niu, Y.; Ke, Q.; Kou, X. Protection of Agarwood Essential Oil Aroma by Nanocellulose-Graft-Polylactic Acid. Int. J. Biol. Macromol. 2021, 183, 743–752. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, J.; Lv, Y.; Yu, J.; Wu, J.; Zhang, J.; He, J. Thermoplastic Cellulose-Graft-Poly(L-Lactide) Copolymers Homogeneously Synthesized in an Ionic Liquid with 4-Dimethylaminopyridine Catalyst. Biomacromolecules 2009, 10, 2013–2018. [Google Scholar] [CrossRef]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From Interfacial Ring-Opening Polymerization to Melt Processing of Cellulose Nanowhisker-Filled Polylactide-Based Nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, W.; Shen, R.; Yan, Y. Emulsion Electrospun PLA/Calcium Alginate Nanofibers for Periodontal Tissue Engineering. J. Biomater. Appl. 2020, 34, 763–777. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Boguń, M.; Mrozińska, Z.; Kaczmarek, A. Physical Properties, Chemical Analysis, and Evaluation of Antimicrobial Response of New Polylactide/Alginate/Copper Composite Materials. Mar. Drugs 2020, 18, 660. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Giełdowska, M.; Mrozińska, Z.; Boguń, M. Poly(Lactic Acid)/Zinc/Alginate Complex Material: Preparation and Antimicrobial Properties. Antibiotics 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber–Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Rees, D.A.; Thom, D. Characterisation of Alginate Composition and Block-Structure by Circular Dichroism. Carbohydr. Res. 1980, 81, 305–314. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Chatzichristidi, M.; Sakellariou, G.; Lohse, D. Graft Copolymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-0-471-44026-0. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular Architectures by Living and Controlled/Living Polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Sakellariou, G. Macromolecular Architectures by Living and Controlled/Living Polymerizations. In Controlled and Living Polymerizations; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 343–443. ISBN 978-3-527-62909-1. [Google Scholar]
- Jamshidi, K.; Hyon, S.-H.; Ikada, Y. Thermal Characterization of Polylactides. Polymer 1988, 29, 2229–2234. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, A. Synthesis and Characterization of Star-Shaped Poly(D,L-Lactide)-Block-Poly(Ethylene Glycol) Copolymers. Polym. Bull. 2010, 65, 883–892. [Google Scholar] [CrossRef]
- Wen, J.; Zhuo, R.-X. Preparation and characterization of poly(D,L-lactide-co-ethylene methyl phosphate). Polym. Int. 1998, 47, 503–509. [Google Scholar] [CrossRef]
- Baśko, M.; Kubisa, P. Cationic Polymerization of L,L-Lactide. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 2650–2658. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Stricker, A. Polylactones 48. SnOct2-Initiated Polymerizations of Lactide: A Mechanistic Study. Macromolecules 2000, 33, 702–709. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and Mechanism of Cyclic Esters Polymerization Initiated with Tin(II) Octoate. 3. Polymerization of L,L-Dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Li, W.; Cao, G.; Chen, W. Kinetics of Thermo-Oxidative and Thermal Degradation of Poly(D,L-Lactide) (PDLLA) at Processing Temperature. Polym. Degrad. Stab. 2006, 91, 3259–3265. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal Degradation of Binary Physical Mixtures and Copolymers of Poly(ε-Caprolactone), Poly(D, L-Lactide), Poly(Glycolide). Polym. Degrad. Stab. 2004, 84, 393–398. [Google Scholar] [CrossRef]
- Cam, D.; Marucci, M. Influence of Residual Monomers and Metals on Poly (L-Lactide) Thermal Stability. Polymer 1997, 38, 1879–1884. [Google Scholar] [CrossRef]
- Fan, Y.; Nishida, H.; Hoshihara, S.; Shirai, Y.; Tokiwa, Y.; Endo, T. Pyrolysis Kinetics of Poly(L-Lactide) with Carboxyl and Calcium Salt End Structures. Polym. Degrad. Stab. 2003, 79, 547–562. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal Behavior of Alginic Acid and Its Sodium Salt. Eclet. Quím. 2004, 29, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene Aerogels Grafted with PMMA: I. Molecular and Interparticle Crosslinking. Soft Matter 2013, 9, 1516–1530. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene Aerogels Grafted with PMMA: II. Nanoscopic Characterization and Origin of Macroscopic Deformation. Soft Matter 2013, 9, 1531–1539. [Google Scholar] [CrossRef]
- Chriti, D.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels 2018, 4, 66. [Google Scholar] [CrossRef] [Green Version]







| Sample | PLA Content (%) |
|---|---|
| g-Ca-alg-10 (G41) | 12 |
| g-Ca-alg-20 (G41) | 17 |
| g-Ca-alg-30 (G41) | 13 |
| g-Ca-alg-40 (G41) | 18 |
| g-Ca-alg-100 (G41) | 17 |
| g-Ca-alg-20 (G56) | 13 |
| g-Ca-alg-40 (G56) | 14 |
| g-Co-alg-20 (G41) | 2 |
| g-Co-alg-40 (G41) | 29 |
| g-Co-alg-20 (G56) | 2 |
| g-Ni-alg-20 (G56) | 2 |
| g-Ni-alg-40 (G56) | 14 |
| g-Cu-alg-20 (G41) | 15 |
| g-Cu-alg-40 (G41) | 29 |
| g-Cu-alg-20 (G56) | 6 |
| Sample a | Bulk Density ρb (g cm−3) | Skeletal Density ρs (g cm−3) | Porosity b Π (% v/v) | BET Surf. Area σ (m2 g−1) (Micropore Surf. Area) c | VTotal d (V1.7–300nm) e (cm3 g−1) | Av. Pore Diam. f (4VTotal/σ) (nm) |
|---|---|---|---|---|---|---|
| Ca-alg (G41) | 0.076 ± 0.006 | 1.97 ± 0.03 | 96 | 340 (53) | 13 (2.0) | 23 (149) |
| g-Ca-alg-10 (G41) | 0.12 ± 0.01 | 1.85 ± 0.02 | 94 | 377 (8) | 8.1 (2.0) | 21 (86) |
| g-Ca-alg-20 (G41) | 0.104 ± 0.006 | 1.80 ± 0.02 | 94 | 433 | 9.1 (1.2) | 12 (84) |
| g-Ca-alg-30 (G41) | 0.10 ± 0.01 | 1.84 ± 0.02 | 94 | 425 | 9.2 (1.2) | 13 (86) |
| g-Ca-alg-40 (G41) | 0.11 ± 0.01 | 1.79 ± 0.02 | 94 | 390 | 8.5 (1.5) | 16 (88) |
| g-Ca-alg-100 (G41) | 0.12 ± 0.01 | 1.80 ± 0.01 | 93 | 398 | 7.8 (1.5) | 16 (78) |
| Ca-alg (G56) | 0.068 ± 0.006 | 1.95 ± 0.02 | 97 | 472 (88) | 14 (1.6) | 15 (120) |
| g-Ca-alg-20 (G56) | 0.08 ± 0.02 | 1.82 ± 0.02 | 96 | 438 (17) | 12 (1.4) | 14 (109) |
| g-Ca-alg-40 (G56) | 0.061 ± 0.005 | 1.81 ± 0.01 | 97 | 422 (12) | 16 (1.1) | 11 (150) |
| Co-alg (G41) | 0.18 ± 0.02 | 1.93 ± 0.01 | 91 | 286 (49) | 5.0 (2.1) | 29 (70) |
| g-Co-alg-20 (G41) | 0.18 ± 0.01 | 1.91 ± 0.02 | 91 | 250 (18) | 5.0 (1.4) | 23 (80) |
| g-Co-alg-40 (G41) | 0.24 ± 0.04 | 1.67 ± 0.01 | 86 | 160 | 3.6 (1.3) | 32 (89) |
| Co-alg (G56) | 0.14 ± 0.01 | 2.05 ± 0.02 | 93 | 289 (54) | 6.6 (1.1) | 15 (92) |
| g-Co-alg-20 (G56) | 0.17 ± 0.03 | 2.04 ± 0.04 | 92 | 229 | 5.4 (0.6) | 12 (94) |
| Ni-alg (G56) | 0.14 ± 0.01 | 1.93 ± 0.01 | 93 | 275 (45) | 6.6 (0.9) | 15 (96) |
| g-Ni-alg-20 (G56) | 0.12 ± 0.02 | 1.91 ± 0.02 | 94 | 254 (21) | 7.8 (0.7) | 12 (123) |
| g-Ni-alg-40 (G56) | 0.12 ± 0.04 | 1.80 ± 0.01 | 93 | 154 (12) | 7.8 (0.8) | 22 (202) |
| Cu-alg (G41) | 0.068 ± 0.006 | 2.06 ± 0.02 | 97 | 568 (84) | 14 (3.7) | 27 (100) |
| g-Cu-alg-20 (G41) | 0.08 ± 0.01 | 1.88 ± 0.02 | 96 | 542 (63) | 12 (3.8) | 28 (88) |
| g-Cu-alg-40 (G41) | 0.09 ± 0.02 | 1.74 ± 0.06 | 95 | 510 (19) | 11 (3.2) | 25 (83) |
| Cu-alg (G56) | 0.056 ± 0.006 | 2.12 ± 0.06 | 97 | 584 (109) | 17 (2.6) | 19 (119) |
| g-Cu-alg-20 (G56) | 0.09 ± 0.01 | 2.03 ± 0.02 | 96 | 494 | 11 (2.9) | 24 (86) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raptopoulos, G.; Choinopoulos, I.; Kontoes-Georgoudakis, F.; Paraskevopoulou, P. Polylactide-Grafted Metal-Alginate Aerogels. Polymers 2022, 14, 1254. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14061254
Raptopoulos G, Choinopoulos I, Kontoes-Georgoudakis F, Paraskevopoulou P. Polylactide-Grafted Metal-Alginate Aerogels. Polymers. 2022; 14(6):1254. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14061254
Chicago/Turabian StyleRaptopoulos, Grigorios, Ioannis Choinopoulos, Filippos Kontoes-Georgoudakis, and Patrina Paraskevopoulou. 2022. "Polylactide-Grafted Metal-Alginate Aerogels" Polymers 14, no. 6: 1254. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14061254