Development of Nanocomposite Materials Based on Conductive Polymers for Using in Glucose Biosensor
Abstract
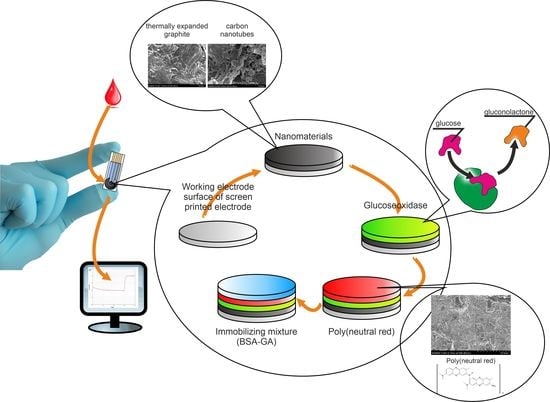
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Conducting Polymers
2.2.1. Electrochemical Polymerization of Aniline on the Surface of the Working Electrode
2.2.2. Electrochemical Polymerization of Neutral Red on the Surface of the Working Electrode
2.2.3. Electrochemical Polymerization of Thionine on the Surface of the Working Electrode
2.3. Formation of Nanocomposite Materials Based on the Obtained Conductive Polymers
2.4. Formation of Working Electrodes Based on Conductive Polymers and Nanocomposite Materials Based on Them
2.5. Electrochemical Measurements
2.6. Impedance Spectroscopy
2.7. IR Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
3. Results
3.1. Formation of Nanocomposite Materials Based on Conductive Polymers
3.2. Electrochemical Properties of Electrodes Modified by Created Nanocomposites
3.3. Practical Use of Biosensors Based on Obtained Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Villalonga, A.; Sánchez, A.; Mayol, B.; Reviejo, J.; Villalonga, R. Electrochemical biosensors for food bioprocess monitoring. Curr. Opin. Food Sci. 2022, 43, 18–26. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.V.; Aleksandrovskaya, A.Y.; Safonov, A.V.; Popova, N.M.; Spitsin, B.V.; Naumova, A.O.; Zaitsev, N.K. Tuning the wetting angle of fluorinated polymer with modified nanodiamonds: Towards new type of biosensors. Mendeleev. Commun. 2020, 30, 453–455. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Liu, L.; Qiao, H. A review of biosensor technology and algorithms for glucose monitoring. J. Diabetes Complicat. 2021, 35, 107929. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, S.; Li, T.; Wang, X.; Jiang, Y.; Zhou, Y.; Zhou, X.; Huang, X.; Liang, P. An electroactive biofilm-based biosensor for water safety: Pollutants detection and early-warning. Biosens. Bioelectron. 2021, 173, 112822. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Arduini, F. The technology tree in the design of glucose biosensors. TrAC Trends Anal. Chem. 2019, 120, 115642. [Google Scholar] [CrossRef]
- Idumah, C.I. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth. Met. 2021, 273, 116674. [Google Scholar] [CrossRef]
- Aleksandrovskaya, A.Y.; Melnikov, P.V.; Safonov, A.V.; Abaturova, N.A.; Spitsyn, B.V.; Naumova, A.O.; Zaitsev, N.K. The Effect of Modified Nanodiamonds on the Wettability of the Surface of an Optical Oxygen Sensor and Biological Fouling During Long-Term in Situ Measurements. Nanotechnologies Russ. 2019, 14, 389–396. [Google Scholar] [CrossRef]
- Pan, H.M.; Gonuguntla, S.; Li, S.; Trau, D. 3.33 Conjugated Polymers for Biosensor Devices; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3, ISBN 9780081006924. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Interfacial composition, structure, and properties of ionic liquids and conductive polymers for the construction of chemical sensors and biosensors: A perspective. Curr. Opin. Electrochem. 2020, 23, 47–56. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Glennon, J.D.; Gedanken, A.; Vashist, S.K. Achievement and assessment of direct electron transfer of glucose oxidase in electrochemical biosensing using carbon nanotubes, graphene, and their nanocomposites. Microchim. Acta 2017, 184, 369–388. [Google Scholar] [CrossRef]
- Feizabadi, M.; Ajloo, D.; Soleymanpour, A.; Faridnouri, H. Study of electron transport in the functionalized nanotubes and their impact on the electron transfer in the active site of horseradish peroxidase. J. Phys. Chem. Solids 2018, 116, 313–323. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Kharkova, A.S.; Kurbanaliyeva, S.K.; Kuznetsova, L.S.; Machulin, A.V.; Tarasov, S.E.; Melnikov, P.V.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. Use of biocompatible redox-active polymers based on carbon nanotubes and modified organic matrices for development of a highly sensitive BOD biosensor. Enzyme Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Yan, Z.; Chen, J. Highly selective and stable glucose biosensor based on incorporation of platinum nanoparticles into polyaniline-montmorillonite hybrid composites. Microchem. J. 2020, 152, 104266. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ragupathy, D. Graphene/Poly(aniline-co-diphenylamine) nanohybrid for ultrasensitive electrochemical glucose sensor. Nano-Structures Nano-Objects 2019, 20, 100390. [Google Scholar] [CrossRef]
- Lee, K.-P.; Komathi, S.; Nam, N.J.; Gopalan, A.I. Sulfonated polyaniline network grafted multi-wall carbon nanotubes for enzyme immobilization, direct electrochemistry and biosensing of glucose. Microchem. J. 2010, 95, 74–79. [Google Scholar] [CrossRef]
- Attar, A.; Emilia Ghica, M.; Amine, A.; Brett, C.M.A. Poly(neutral red) based hydrogen peroxide biosensor for chromium determination by inhibition measurements. J. Hazard. Mater. 2014, 279, 348–355. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Khar’kova, A.S.; Abramova, T.N.; Kuznetsova, L.S.; Ilyukhina, A.S.; Zaitsev, M.G.; Machulin, A.V.; Reshetilov, A.N. A Hybrid Redox-Active Polymer Based on Bovine Serum Albumin, Ferrocene, Carboxylated Carbon Nanotubes, and Glucose Oxidase. J. Anal. Chem. 2020, 75, 1189–1200. [Google Scholar] [CrossRef]
- Mazar, F.M.; Alijanianzadeh, M.; Molaeirad, A.; Heydari, P. Development of Novel Glucose oxidase Immobilization on Graphene/Gold nanoparticles/Poly Neutral red modified electrode. Process Biochem. 2017, 56, 71–80. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Poly(brilliant green) and poly(thionine) modified carbon nanotube coated carbon film electrodes for glucose and uric acid biosensors. Talanta 2014, 130, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au doped poly-thionine and poly-m-Cresol purple: Synthesis and their application in simultaneously electrochemical detection of two lung cancer markers CEA and CYFRA21-1. Talanta 2021, 224, 121816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kan, X. A boronic acid carbon nanodots/poly(thionine) sensing platform for the accurate and reliable detection of NADH. Bioelectrochemistry 2019, 130, 107344. [Google Scholar] [CrossRef]
- Dalkıran, B.; Fernandes, I.P.G.; David, M.; Brett, C.M.A. Electrochemical synthesis and characterization of poly(thionine)-deep eutectic solvent/carbon nanotube–modified electrodes and application to electrochemical sensing. Microchim. Acta 2020, 187, 609. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M.A. A novel nanostructured poly(thionine)-deep eutectic solvent/CuO nanoparticle film-modified disposable pencil graphite electrode for determination of acetaminophen in the presence of ascorbic acid. Anal. Bioanal. Chem. 2021, 413, 1149–1157. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Kamanin, S.S.; Kharkova, A.S.; Arlyapov, V.A. Glucose biosensor based on screen-printed electrode modified with silicone sol–gel conducting matrix containing carbon nanotubes. 3 Biotech 2019, 9, 290. [Google Scholar] [CrossRef]
- Hamid, H.H.; Harb, M.E.; Elshaer, A.M.; Erahim, S.; Soliman, M.M. Electrochemical preparation and electrical characterization of polyaniline as a sensitive biosensor. Microsyst. Technol. 2018, 24, 1775–1781. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Barsan, M.; Brett, C.M.A. Characterisation of poly(neutral red) modified carbon film electrodes; Application as a redox mediator for biosensors. J. Solid State Electrochem. 2007, 11, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Gorshenev, V.N.; Bibikov, S.B.; Novikov, Y.N. Conducting materials based on thermally expanded graphite. Russ. J. Appl. Chem. 2003, 76, 603–606. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalessky, S.S.; Ananikov, V.P. Comprehensive study of the structure and mechanisms of obtaining and converting gaseous, liquid and solid chemical systems by mass spectrometry, NMR spectroscopy and electron microscopy. Adv. Chem. 2013, 82, 648–685. [Google Scholar]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarrintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive polymers in water treatment: A review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Kumari, A.; Rajeev, R.; Benny, L.; Sudhakar, Y.N.; Varghese, A.; Hegde, G. Recent advances in carbon nanotubes-based biocatalysts and their applications. Adv. Colloid Interface Sci. 2021, 297, 102542. [Google Scholar] [CrossRef]
- Kim, H.S.; Bae, H.S.; Yu, J.; Kim, S.Y. Thermal conductivity of polymer composites with the geometrical characteristics of graphene nanoplatelets. Sci. Rep. 2016, 6, 26825. [Google Scholar] [CrossRef] [Green Version]
- Reshetilov, A.N.; Plekhanova, Y.V.; Tarasov, S.E.; Arlyapov, V.A.; Kolesov, V.V.; Gutorov, M.A.; Gotovtsev, P.M.; Vasilov, R.G. Influence of some carbon nanomaterials on the oxidation of ethyl alcohol by bacterial cells of Gluconobacter oxydans. Appl. Biochem. Microbiol. 2017, 53, 115–122. [Google Scholar] [CrossRef]
- Ramesh, P.; Sampath, S. Electrochemical and spectroscopic characterization of quinone functionalized exfoliated graphite. Analyst 2001, 126, 1872–1877. [Google Scholar] [CrossRef]
- Calas-Blanchard, C.; Noguer, T.; Comtat, M.; Mauran, S.; Marty, J.-L. Potentialities of expanded natural graphite as a new transducer for NAD+-dependent dehydrogenase amperometric biosensors. Anal. Chim. Acta 2003, 484, 25–31. [Google Scholar] [CrossRef]
- Budi, S.; Ayuningsih, A.; Pratiwi, C.; Paristiowati, M.; Fadiran, R.; Sugihartono, I. Electropolymerization of Polyaniline Film as a Conductive Layer for the Electrodeposition of NiCo Alloy. J. Phys. Conf. Ser. 2020, 1428, 012017. [Google Scholar] [CrossRef]
- Yang, C.; Yi, J.; Tang, X.; Zhou, G.; Zeng, Y. Studies on the spectroscopic properties of poly(neutral red) synthesized by electropolymerization. React. Funct. Polym. 2006, 66, 1336–1341. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Development of Novel Glucose and Pyruvate Biosensors at Poly(Neutral Red) Modified Carbon Film Electrodes. Application to Natural Samples. Electroanalysis 2006, 18, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Mahbubur Rahman, M.; Lee, J.-J. Sensitivity control of dopamine detection by conducting poly(thionine). Electrochem. Commun. 2021, 125, 107005. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; García-Mendiola, T.; Enebral-Romero, E.; del Caño, R.; Vera-Hidalgo, M.; Vázquez Sulleiro, M.; Navío, C.; Pariente, F.; Pérez, E.M.; Lorenzo, E. A MoS2 platform and thionine-carbon nanodots for sensitive and selective detection of pathogens. Biosens. Bioelectron. 2021, 189, 113375. [Google Scholar] [CrossRef] [PubMed]
- Topçu, E.; Alanyalioǧlu, M. Electrochemical formation of poly(thionine) thin films: The effect of amine group on the polymeric film formation of phenothiazine dyes. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Mohammad, H.; Stepashkin, A.A.; Tcherdyntsev, V. V Effect of Graphite Filler Type on the Thermal Conductivity and Mechanical Behavior of Polysulfone-Based Composites. Polymers 2022, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, J.; Wang, L. Electrochemical synthesis of a nanocomposite consisting of carboxy-modified multi-walled carbon nanotubes, polythionine and platinum nanoparticles for simultaneous voltammetric determination of myricetin and rutin. Microchim. Acta 2018, 185, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Kirihara, K.; Okigawa, Y.; Hasegawa, M.; Ding, W.; Wang, Y.; Mukaida, M.; Zhou, Y.; Wei, Q. Extracting carrier mobility using a photoinduced charge transfer reaction: From conducting polymers to nanocarbon materials. Org. Electron. 2020, 78, 105615. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Madrakian, T.; Afkhami, A.; Bagheri, H. Spectroelectrochemical and electrochromic behavior of poly(methylene blue) and poly(thionine)-modified multi-walled carbon nanotubes. J. Solid State Electrochem. 2021, 25, 1217–1229. [Google Scholar] [CrossRef]
- Laviron, E. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Coutinho, I.; Fortunato, E. A Simple Procedure to Fabricate Paper Biosensor and Its Applicability—NADH/NAD+ Redox System. J. Pharm. Pharmacol. 2018, 6, 175–187. [Google Scholar]
- Fátima Giarola, J.; Mano, V.; Pereira, A.C. Development and application of a voltammetric biosensor based on Polypyrrole/uricase/graphene for uric acid determination. Electroanalysis 2018, 30, 119–127. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Ramírez-Delgado, V.; Cruz-Ramirez, M.; Hernández-Ayala, L.F.; Reyes-Vidal, Y.; Patakfalvi, R.; García-Ramos, J.C.; Tenorio, F.J.; Ruiz-Azuara, L.; Ortiz-Frade, L. The role of the π acceptor character of polypyridine ligands on the electrochemical response of Co (II) complexes and its effect on the homogenous electron transfer rate constant with the enzyme glucose oxidase. J. Mex. Chem. Soc. 2015, 59, 282–293. [Google Scholar]
- Sekretaryova, A.N.; Vagin, M.Y.; Beni, V.; Turner, A.P.F.; Karyakin, A.A. Unsubstituted phenothiazine as a superior water-insoluble mediator for oxidases. Biosens. Bioelectron. 2014, 53, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A mediator microbial biosensor for assaying general toxicity. Enzyme Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Finaenov, A.I.; Zabud’Kov, S.L.; Yakovleva, E. V Thermally expanded graphite: Synthesis, properties, and prospects for use. Russ. J. Appl. Chem. 2006, 79, 1741–1751. [Google Scholar] [CrossRef]
- Plekhanova, Y.; Tarasov, S.; Kitova, A.; Kolesov, V.; Kashin, V.; Sundramoorthy, A.K.; Reshetilov, A. Modification of thermally expanded graphite and its effect on the properties of the amperometric biosensor. 3 Biotech 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlyapov, V.A.; Kuznetsova, L.S.; Kharkova, A.S.; Provotorova, D.V.; Nenarochkina, E.D.; Kamanina, O.A.; Machulin, A.V.; Ponamoreva, O.N.; Alferova, V.A.; Reshetilov, A.N. On the Development of Reagent-Free Conductive Nanocomposite Systems for the Modification of Printed Electrodes when Producing Glucose Biosensors. Nanobiotechnol. Rep. 2022, 17, 106–117. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-arporn, R.P. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater. Sci. Eng. C 2017, 76, 398–405. [Google Scholar] [CrossRef] [PubMed]









| Matrix | Operating Potential, mV | Heterogeneous Rate Constant, (s−1·cm) | Rate Constant of Matrix Interaction with GOx, cm3/(mol·s) |
|---|---|---|---|
| pNR | −550 | 0.35 ± 0.02 | 14 ± 2 |
| pNR-TEG | −550 | 1.43 ± 0.03 | 13 ± 3 |
| pNR-CNTs | −550 | 0.90 ± 0.05 | 11 ± 3 |
| pTN | 350 | 0.90 ± 0.02 | 5.9 ± 0.3 |
| pTN-TEG | 350 | 1.61 ± 0.08 | 6.2 ± 0.3 |
| pTN-CNTs | 350 | 0.14 ± 0.01 | 6.1 ± 0.3 |
| PANI | 450 | 0.46 ± 0.06 | 0.1 ± 0.01 |
| PANI-TEG | 450 | 0.57 ± 0.09 | 0.1 ± 0.01 |
| PANI-CNTs | 450 | 0.55 ± 0.07 | 0.1 ± 0.01 |
| Conductive Matrix | Range of Determined Concentrations, mmol/dm3 | Sensitivity Coefficient nA × dm3/mmol | Detection Limit (Cmin) mmol/dm3 | Time of a Single Measurement, min |
|---|---|---|---|---|
| pNR | 0.09–1.0 | 62 ± 4 | 0.03 | 1–2 |
| pNR-TEG | 0.006–0.5 | 1000 ± 200 | 0.002 | 1–2 |
| pNR-CNT | 0.02–4.2 | 220 ± 10 | 0.007 | 1–2 |
| pTN | 0.25–1.9 | 165 ± 3 | 0.08 | 1 |
| pTN-TEG | 0.06–0.62 | 450 ± 20 | 0.02 | 1 |
| pTN-CNT | 0.2–1.7 | 125 ± 2 | 0.07 | 1 |
| PANI | 0.5–0.72 | 11 ± 1 | 0.15 | 1–2 |
| PANI-TEG | 0.4–1.4 | 23 ± 5 | 0.12 | 1–2 |
| PANI-CNT | 1.5–2.1 | 17 ± 2 | 0.23 | 1–2 |
| BSA covalently linked to ferrocene and containing CNT-NH2 [63] | 0.1–1.8 | 330 ± 10 | – | 1–3 |
| A conjugate of reduced graphene oxide and Fe3O4 nanoparticles [64] | 0.05–1.0 | 5900 | – | – |
| Conducting hydrogel based on organic-inorganic hybrid sol-gel matrix and CNT [29] | 0.045–1.04 | 1480 ± 30 | – | 1–3 |
| Platinum nanoparticles incorporated into PANI-montmorillonite hybrid composites [18] | 0.01–1.94 | – | 0.0001 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, L.S.; Arlyapov, V.A.; Kamanina, O.A.; Lantsova, E.A.; Tarasov, S.E.; Reshetilov, A.N. Development of Nanocomposite Materials Based on Conductive Polymers for Using in Glucose Biosensor. Polymers 2022, 14, 1543. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14081543
Kuznetsova LS, Arlyapov VA, Kamanina OA, Lantsova EA, Tarasov SE, Reshetilov AN. Development of Nanocomposite Materials Based on Conductive Polymers for Using in Glucose Biosensor. Polymers. 2022; 14(8):1543. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14081543
Chicago/Turabian StyleKuznetsova, Lyubov S., Vyacheslav A. Arlyapov, Olga A. Kamanina, Elizaveta A. Lantsova, Sergey E. Tarasov, and Anatoly N. Reshetilov. 2022. "Development of Nanocomposite Materials Based on Conductive Polymers for Using in Glucose Biosensor" Polymers 14, no. 8: 1543. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14081543