Performance Evaluation of Nonacosan-10-ol-Based Polyethylene Packaging Material Using Molecular Dynamics Simulations
Abstract
:1. Introduction
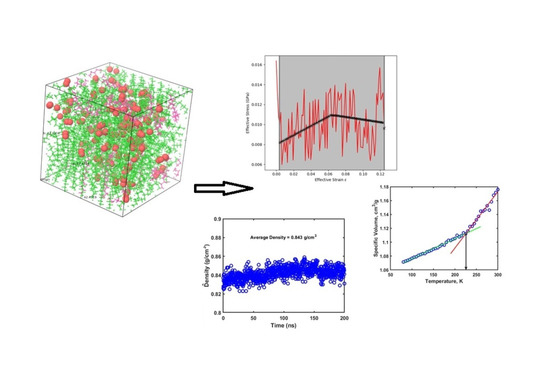
2. Simulations Details
- Brownian dynamics for 0.1 ns with NVT ensemble at 10 K.
- MD simulation with a time step of 1 fs with NVT ensemble at 300 K.
- MD simulation with a time step of 1 fs with NVT ensemble at 700 K.
- MD simulation with a time step of 2 fs with NPT ensemble at 300 K and 1.01325 bar.
- MD simulation for 10 ns with NPT ensemble at 300 K and 1013.25 bar.
- MD simulation for 10 ns with NPT ensemble at 300 K and 1.01325 bar.
- Brownian minimization for 100 ps.
- MD simulation for 200 ns with NPT ensemble at 300 K and 1.01325 bar.
- Analysis of bulk properties for the final system.
3. Results and Discussion
3.1. Stress-Strain Results
3.2. Glass Transition Temperature (Tg) Results
3.3. Diffusion Coefficient Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, Z.; Luo, C.; Guo, Y.; Ahmed, I.; Pavase, T.R.; Lv, L.; Li, Z.; Lin, H. Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets. Food Packag. Shelf Life 2019, 20, 100320. [Google Scholar] [CrossRef]
- Parisi, S.; Hu, M.; Jacobsen, C. (Eds.) Oxidative stability and shelf life of foods containing oils and fats. Anal. Bioanal. Chem. 2016, 408, 7549–7550. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J. Biomimetic polyphenol coatings for antioxidant active packaging applications. Colloid Interface Sci. Commun. 2016, 13, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene Films Containing Plant Extracts in the Polymer Matrix as Antibacterial and Antiviral Materials. Int. J. Mol. Sci. 2021, 22, 13438. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; George, B.; Shankar, S.; Dennis, T.; Wacławek, S.; Černík, M.; Padil, V. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Popović, S.; Hromiš, N.; Šuput, D.; Bulut, S.; Vitas, S.; Savić, M.; Lazić, V. Pumpkin seed oil cake/polyethylene film as new food packaging material, with perspective for packing under modified atmosphere. Packag. Technol. Sci. 2020, 34, 25–33. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, B.; Todosijević, M.; Đorđević, I.; Stanković, J.; Mitić, Z.; Tešević, V.; Marin, P. Nonacosan-10-ol and n-Alkanes in Leaves of Pinus pinaster. Nat. Prod. Commun. 2020, 15, 1–4. [Google Scholar] [CrossRef]
- Fernandes, S.; Madhuranthakam, C. Molecular Dynamics Simulation of a Superhydrophobic Cellulose Derivative Targeted for Eco-Friendly Packaging Material. Macromol. Theory Simul. 2020, 30, 2000056. [Google Scholar] [CrossRef]
- OPLS4|Schrödinger. Schrodinger.com, 2022. Available online: https://www.schrodinger.com/products/opls4 (accessed on 4 April 2022).
- Hossain, D.; Tschopp, M.A.; Ward, D.K.; Bouvard, J.L.; Wang, P.; Horstemeyer, M.F. Molecular dynamics simulations of deformation mechanisms of amorphous polyethylene. Polymer 2010, 51, 6071–6083. [Google Scholar] [CrossRef]
- (10S)-Nonacosan-10-ol. Chemsrc.com, 2022. Available online: https://www.chemsrc.com/en/cas/504-55-2_340153.html (accessed on 4 April 2022).
- Djoković, V.; Mtshali, T.; Luyt, A. The influence of wax content on the physical properties of low-density polyethylene-wax blends. Polym. Int. 2003, 52, 999–1004. [Google Scholar] [CrossRef]
- Fakirov, S.; Krasteva, B. On the Glass Transition Temperature of Polyethylene as Revealed by Microhardness Measurements. J. Macromol. Sci. Part B 2000, 39, 297–301. [Google Scholar] [CrossRef]
- Hosoya, R.; Morita, H.; Nakajima, K. Analysis of Nanomechanical Properties of Polyethylene Using Molecular Dynamics Simulation. Macromolecules 2020, 53, 6163–6172. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.; He, Z.; Lan, F.; Liu, H. The glass transition temperature measurements of polyethylene: Determined by using molecular dynamic method. RSC Adv. 2016, 6, 12053–12060. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, G.; Yang, S. Molecular Simulation of Oxygen Diffusion in Polymers. In Proceedings of the 17th IAPRI World Conference on Packaging, Tianjin, China, 12–15 October 2010; pp. 212–216. [Google Scholar]









| Weight % of PE- Nonacosan-10-ol | Diffusion Coefficient D, m2/s | % Decrease in D with Respect to PE |
| 100–0 | 5.062 × 10−10 ± 1.2067 × 10−11 | - |
| 94.8–5.2 | 4.7158 × 10−10 ± 1.089 × 10−11 | 6.83 |
| 82.9–17.1 | 4.6547 × 10−10 ± 1.6711 × 10−11 | 8.05 |
| 70.8–29.2 | 4.2289 × 10−10 ± 1.5893 × 10−11 | 16.46 |
| 59.2–40.8 | 4.3856 × 10−10 ± 9.5663 × 10−12 | 13.36 |
| 54.8–45.2 | 4.3060 × 10−10 ± 7.6644 × 10−12 | 14.94 |
| 0–100 | 1.0159 × 10−9 ± 1.7738 × 10−11 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhuranthakam, C.M.R.; Pandiyan, S.; Penlidis, A. Performance Evaluation of Nonacosan-10-ol-Based Polyethylene Packaging Material Using Molecular Dynamics Simulations. Polymers 2022, 14, 1779. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091779
Madhuranthakam CMR, Pandiyan S, Penlidis A. Performance Evaluation of Nonacosan-10-ol-Based Polyethylene Packaging Material Using Molecular Dynamics Simulations. Polymers. 2022; 14(9):1779. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091779
Chicago/Turabian StyleMadhuranthakam, Chandra Mouli R., Sudharsan Pandiyan, and Alexander Penlidis. 2022. "Performance Evaluation of Nonacosan-10-ol-Based Polyethylene Packaging Material Using Molecular Dynamics Simulations" Polymers 14, no. 9: 1779. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091779