Novel Mesogenic Vinyl Ketone Monomers and Their Based Polymers
Abstract
:1. Introduction

2. Experimental
2.1. Materials and Methods
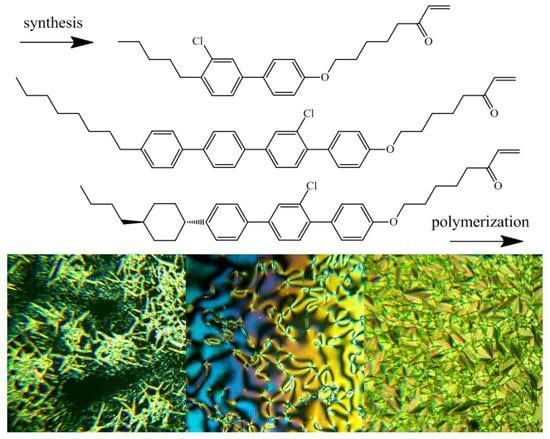
2.2. Monomer Synthesis
2.3. Polymer Synthesis
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Monomers and Their Phase Behavior
- -
- the synthesis of the corresponding cyclopropan-1-ols from the esters using the Kulinkovich reaction;
- -
- cycle splitting followed by its transformation to the corresponding substituted 2-bromoethyl ketone;
- -
3.2. Synthesis of Polymers and Their Phase Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyons, A.R. Polymerization of vinyl ketones. J. Polym. Sci. Macromol. Rev. 1972, 6, 251–293. [Google Scholar] [CrossRef]
- Schildknecht, C.E.; Zoss, A.O.; Grosser, F. Ionic Polymerization of Some Vinyl Compounds. Ind. Eng. Chem. 1949, 41, 2891–2896. [Google Scholar] [CrossRef]
- Thomas, P.R.; Tyler, G.J.; Edwards, T.E.; Radcliffe, A.T.; Cubbon, R.C.P. The anionic polymerization of some alkyl vinyl ketones. Polymer 1964, 5, 525–531. [Google Scholar] [CrossRef]
- Mulvaney, J.E.; Dillon, J.G. Anionic polymerization and copolymerization of acrylophenone. J. Polym. Sci. A-1 1968, 6, 1849–1855. [Google Scholar] [CrossRef]
- Pieroni, O.; Ciardelli, F.; Botteghi, C.; Lardicci, L.; Salvadori, P.; Pino, P. Optically Active Vinyl Polymers. XVIII: Polymerization of Optically Active Alkyl Vinyl Ketones. J. Polym. Sci. C 1969, 22, 993–1007. [Google Scholar] [CrossRef]
- Brown, F.; Berardinelli, F.; Kray, R.J.; Rosen, L.J. Polymerization of Methyl Isopropenyl Ketone. Ind. Eng. Chem. 1959, 51, 79–82. [Google Scholar] [CrossRef]
- Marvel, C.S.; Casey, D.J. Preparation of Polymers Containing Pyridine Units from Polyvinyl Ketones. J. Org. Chem. 1959, 24, 957–963. [Google Scholar] [CrossRef]
- Tsuiuta, T.; Fujio, R.; Furukawa, J. Anionic polymerization of vinyl ketones. Makromol. Chem. 1964, 80, 172–184. [Google Scholar]
- Matsuda, T.; Yamakita, H.; Fujii, S. Radiation Induced Polymerization of Methyl Vinyl Ketone. Kobunshi Kagaku 1964, 21, 415–420. [Google Scholar] [CrossRef]
- Marvel, C.S.; Riddle, E.H.; Corner, J.O. Structure of Vinyl Polymers. XII. The Polymer of Methyl Isopropenyl Ketone. J. Am. Chem. Soc. 1942, 64, 92–94. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Lay, T.C. Polymerization of methyl isopropenyl ketone and coloration of the polymers. Makromol. Chem. 1967, 110, 185–196. [Google Scholar] [CrossRef]
- Yongjun, J.I.; Jianguo, Y.A.N.G. Synthesis of Methyl Isopropyl Ketone and Diethyl Ketone over Ni-Na/ZrO2-MnO2-ZnO Catalyst. Chin. J. Chem. Eng. 2011, 19, 656–660. [Google Scholar]
- Acosta, J.L.; Sastre, R.; Garcia, M.; Fontan, J. Radical Copolymerization of Vinyl Ketones. I. Copolymerization with Acrylonitrile. Polym. J. 1976, 8, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Dan, E.; Guillet, J.E. Photochemistry of Ketone Polymers. X. Chain Scission Reaction in the Solid State. Macromolecules 1973, 6, 230–235. [Google Scholar] [CrossRef]
- Wissbrun, K.F. The Photolysis of Polymethylvinyl Ketone and Polymethyl Isopropenyl Ketone. J. Am. Chem. Soc. 1959, 81, 58–62. [Google Scholar] [CrossRef]
- Guillet, J.E.; Norrish, R.G.W. Photolysis of Poly Methyl Vinyl Ketone: Formation of Block Polymers. Nature 1954, 173, 625–627. [Google Scholar] [CrossRef]
- Small, R.D., Jr.; Scaiano, J.C. Importance of Intermolecular Biradical Reactions in Polymer Photochemistry. Poly(phenyl vinyl ketone). Macromolecules 1978, 11, 840–841. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Stewart, L.C. The triplet lifetime of poly(phenyl vinyl ketone). A laser flash photolysis study. Polymer 1982, 23, 913–915. [Google Scholar] [CrossRef]
- Guillet, J. Environmental aspects of photodegradable plastics. Macromol. Symp. 1997, 123, 209–224. [Google Scholar] [CrossRef]
- Sugita, K. Application of photodegradable polymers to imaging and microfabrication technologies: A review of recent research papers in the last 4 years. Prog. Org. Coat. 1997, 31, 87–95. [Google Scholar] [CrossRef]
- Green, O.; Smith, N.A.; Ellis, A.B.; Burstyn, J.N. AgBF4-Impregnated Poly(vinyl phenyl ketone): An Ethylene Sensing Film. J. Am. Chem. Soc. 2004, 126, 5952–5953. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, S.; Park, S.-Y.; Chung, I. Biodegradable PCL-b-PLA Microspheres with Nanopores Prepared via RAFT Polymerization and UV Photodegradation of Poly(Methyl Vinyl Ketone) Blocks. Polymers 2021, 13, 3964. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, J.; Yoon, J.-Y.; Chung, I. Fabrication and photodegradable properties of chitosan-g-poly(methyl vinyl ketones) nanoporous microspheres. Mol. Cryst. Liq. Cryst. 2017, 654, 53–61. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; Giebel, E.; Frank, E.; Müller, A.; Ackermann, L.-M.; Tironi, C.N.; Mourgas, G.; Unold, J.; Klapper, M.; Buchmeiser, M.R.; et al. Poly(methyl vinyl ketone) as a Potential Carbon Fiber Precursor. Chem. Mater. 2017, 29, 780–788. [Google Scholar] [CrossRef]
- Marvel, C.S.; Levesqu, C.L. The Structure of Vinyl Polymers: The Polymer from Methyl Vinyl Ketone. J. Am. Chem. Soc. 1938, 60, 280–284. [Google Scholar] [CrossRef]
- Jones, T.T.; Melville, H.W. The photochemical polymerization of methyl vinyl ketone vapour. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1946, 187, 19–36. [Google Scholar]
- Zhao, Y.; Liu, X.; Liu, Y.; Wu, Z.; Zhao, X.; Fu, X. When CMRP met alkyl vinyl ketone: Visible light induced living radical polymerization (LRP) of ethyl vinyl ketone (EVK). Chem. Commun. 2016, 52, 12092–12095. [Google Scholar] [CrossRef]
- Mittal, A.; Sivaram, S.; Baskaran, D. Unfavorable Coordination of Copper with Methyl Vinyl Ketone in Atom Transfer Radical Polymerization. Macromolecules 2006, 39, 5555–5558. [Google Scholar] [CrossRef]
- Hepperle, J.A.M.; Luftmann, H.; Studer, A. Controlled Nitroxide-Mediated Radical Polymerization of Methyl and Phenyl Vinyl Ketone. J. Polym. Sci. A Polym. Chem. 2012, 50, 2150–2160. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, G.; Khoshdel, E.; Wooley, K.L. Well-Defined Vinyl Ketone-Based Polymers by Reversible Addition-Fragmentation Chain Transfer Polymerization. J. Am. Chem. Soc. 2007, 129, 10086–10087. [Google Scholar] [CrossRef]
- Guo, R.; Mei, P.; Zhong, Q.; Yao, Y.; Su, Q.; Zhang, J. Well-defined triblock copolymers with a photolabile middle block of poly(phenyl vinyl ketone): Facile synthesis, chain-scission mechanism and controllable photocleavability. RSC Adv. 2015, 5, 31365. [Google Scholar] [CrossRef]
- Lee, I.-H.; Discekici, E.H.; Anastasaki, A.; de Alaniz, J.R.; Hawker, C.J. Controlled radical polymerization of vinyl ketones using visible light. Polym. Chem. 2017, 8, 3351–3356. [Google Scholar] [CrossRef]
- Mueller, A.H.E.; Matyjaszewski, K. (Eds.) Controlled and Living Polymerizations: From Mechanisms to Materials; Wiley-VCH: Weinheim, Germany, 2009; 612p. [Google Scholar]
- Moad, G.; Rizzardo, E. (Eds.) RAFT Polymerization: Methods, Synthesis and Applications; Wiley-VCH GmbH: Weinheim, Germany, 2021; Volume 2, 1240p. [Google Scholar]
- Reeves, J.A.; De Alwis Watuthanthrige, N.; Boyer, C.; Konkolewicz, D. Intrinsic and Catalyzed Photochemistry of Phenylvinylketone for Wavelength Sensitive Controlled Polymerization. ChemPhotoChem 2019, 3, 1171–1179. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Ye, X.; Hayama, H.; Sugino, H.; Nakano, H.; Nakano, T. π-Stacked poly(vinyl ketone)s with accumulated push–pull triphenylamine moieties in the side chain. Polym. Chem. 2017, 8, 708–714. [Google Scholar] [CrossRef]
- Sultane, P.R.; Bielawski, C.W. Stereoelectronically Directed Photodegradation of Poly(adamantyl Vinyl Ketone). Macromol. Rapid Commun. 2019, 40, 1900302. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, G.N.; Karpov, O.N.; Shandryuk, G.A.; Finko, A.V.; Derikov, Y.I.; Mikhalyonok, S.G.; Bezborodov, V.S.; Talroze, R.V. Role of terphenyl-containing carboxylic acid on oligomerization of aryl vinyl ketone. Phys. Chem. Chem. Phys. 2022, 24, 26083–26093. [Google Scholar] [CrossRef]
- Bezborodov, V.S.; Finko, A.V.; Mikhalyonok, S.G.; Derikov, Y.I.; Shandryuk, G.A.; Kuz’menok, N.M.; Arol, A.S.; Karpov, O.N.; Talroze, R.V. Synthesis of new mesomorphic terphenyl derivatives: The influence of terphenylene and functional fragments on the mesomorphic properties and ligand exchange on quantum dots. Liq. Cryst. 2021, 48, 1544–1554. [Google Scholar] [CrossRef]
- Bezborodov, V.S.; Mikhalyonok, S.G.; Kuz’menok, N.M.; Lapanik, V.I.; Sasnouski, G.M. Polyfunctional intermediates for the preparation of liquid-crystalline and anisotropic materials. Liq. Cryst. 2015, 42, 1124–1138. [Google Scholar] [CrossRef]














| Monomer | 1H NMR (400 MHz, CDCl3) δ (ppm) | 13C NMR {H} (101 MHz, CDCl3) δ (ppm) |
|---|---|---|
| BVK | 0.95 (t, J = 7.0 Hz, 3H, Me), 1.39–1.43 (m, 4H, CH2), 1.53–1.59 (m, 2H, CH2), 1.64–1.69 (m, 2H, CH2), 1.71–1.79 (m, 2H, CH2), 1.83–1.90 (m, 2H, CH2), 2.67 (t, J = 7.3 Hz, 2H, CH2), 2.75–2.78 (m, 2H, CH2), 4.03 (t, J = 6.4 Hz, 2H, CH2), 5.86 (dd, J = 10.5, 1.1 Hz, 1H, CHVinyl), 6.26 (dd, J = 17.7, 1.1 Hz, 1H, CHVinyl), 6.40 (dd, J = 17.7, 10.5 Hz, 1H, CHVinyl), 6.97 (d, J = 8.7 Hz, 2H, HAr), 7.29 (d, J = 4.9 Hz, 1H, HAr), 7.39 (dd, J = 7.9, 1.9 Hz, 1H, HAr), 7.50 (d, J = 8.7 Hz, 2H, HAr), 7.56 (d, J = 1.8 Hz, 1H, HAr) | 14.2 (Me), 22.7 (CH2), 23.8 (CH2), 25.9 (CH2), 29.2 (CH2), 29.7 (CH2), 31.7 (CH2), 33.4 (CH2-CAr), 39.6 (CH2-C(O)-CH), 67.9 (CH2-O-CAr), 114.9 (2CAr), 125.0 (CH2Vinyl), 127.5 (CAr), 128.0 (2CAr), 128.2 (CAr), 130.7 (CAr), 132.3 (CAr), 134.4 (CAr), 136.7 (CHVinyl), 138.7 (CAr), 140.1 (CAr), 158.9 (CAr-O-CH2), 200.9 (C=O) |
| QVK | 0.86–0.91 (m, 5H, CH3 + CH2), 1.26–1.43 (m, 8H, 4 × CH2), 1.50–1.58 (m, 2H, CH2), 1.63–1.77 (m, 4H, 2 × CH2), 1.82–1.89 (m, 2H, CH2), 2.63–2.68 (m, 4H, 2 × CH2), 4.03 (t, J = 6.3 Hz, 2H, CH2), 5.84 (d, J = 10.5 Hz, 1H, CHVinyl), 6.24 (d, J = 17.7 Hz, 1H, CHVinyl), 6.38 (dd, J = 17.7, 10.5 Hz, 1H, CHVinyl), 6.98 (d, J = 8.7 Hz, 2H, HAr), 7.28 (d, J = 7.9 Hz, 2H, HAr), 7.40–7.45 (m, 3H, HAr), 7.56–7.58 (m, 3H, HAr), 7.68 (s, 4H, HAr), 7.75 (d, J = 1.6 Hz, 1H, HAr) | 14.3 (Me), 22.8 (CH2), 23.8 (CH2), 25.9 (CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 31.7 (CH2), 32.0 (CH2), 35.8 (CH2-CAr), 39.6 (CH2-C(O)-CH), 67.8 (CH2-O-CAr), 114.2 (2CAr), 125.5 (CH2Vinyl), 127.0 (2CAr), 127.4 (2CAr), 127.6 (2CAr), 128.2 (CAr), 128.4 (CAr), 129.1 (2CAr), 130.8 (2CAr), 131.4 (CAr), 131.8 (CAr), 133.1 (CAr), 136.7 (CHVinyl), 137.9 (CAr), 139.0 (CAr), 140.8 (CAr), 141.0 (CAr), 142.6 (CAr), 158.8 (CAr-O-CH2), 200.9 (C=O) |
| Monomer | Conditions | CAIBN, mol % | RAFT Agent | Conversion, % | Mn, kg·mol−1 | Đ |
|---|---|---|---|---|---|---|
| BVK | in bulk | 0.05 0.4 0.4 0.4 | – – CPDTC DoPAT | 68 65 27 44 | 56.8 32.2 4.6 5.4 | 1.9 2.1 1.11 1.08 |
| 1,4-dioxane | 0.4 0.4 | – DoPAT | 93 87 | 32.3 9.0 | 3.0 1.14 | |
| TVK | in bulk | 0.4 4.0 | – – | 46 39 | 43.8 42.0 | 1.38 1.43 |
| 1,4-dioxane | 0.1 | CDTPC | 22 | 3.5 | 1.09 | |
| QVK | in bulk | 0.4 2.0 | – – | 26 31 | 23.9 24.5 | 1.41 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derikov, Y.I.; Belousov, D.R.; Finko, A.V.; Shandryuk, G.A.; Kuz’menok, N.M.; Mikhalyonok, S.G.; Bezborodov, V.S.; Chernikova, E.V.; Talroze, R.V. Novel Mesogenic Vinyl Ketone Monomers and Their Based Polymers. Polymers 2023, 15, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15010005
Derikov YI, Belousov DR, Finko AV, Shandryuk GA, Kuz’menok NM, Mikhalyonok SG, Bezborodov VS, Chernikova EV, Talroze RV. Novel Mesogenic Vinyl Ketone Monomers and Their Based Polymers. Polymers. 2023; 15(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15010005
Chicago/Turabian StyleDerikov, Yaroslav I., Daniil R. Belousov, Alexander V. Finko, Georgii A. Shandryuk, Nina M. Kuz’menok, Sergei G. Mikhalyonok, Vladimir S. Bezborodov, Elena V. Chernikova, and Raisa V. Talroze. 2023. "Novel Mesogenic Vinyl Ketone Monomers and Their Based Polymers" Polymers 15, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15010005