In Situ Redox Synthesis of Highly Stable Au/Electroactive Polyimide Composite and Its Application on 4-Nitrophenol Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Synthesis of Amino-Capped Aniline Trimer (ACAT)
2.3. Synthesis of Amino-Capped Aniline Pentamer (ACAP)
2.4. Synthesis of Electroactive Polyimide (EPI-5)
2.5. Preparation of Series of Au/Electroactive Polyimide (Au/EPI-5) Composite
2.6. Electrochemical Cyclic Voltammetry of EPI-5 and Series of Au/EPI-5 Composite
2.7. Catalytic Activity
3. Results
3.1. Characterization of ACAT, ACAP, EPAA-5, and EPI-5
3.2. Chemical Oxidation of EPI-5
3.3. Chemical Structural and Morphological Characterization of EPI-5 and Series Au/EPI-5
3.3.1. Characterization of EPI-5 and Series of Au/EPI-5 Composites by FT-IR
3.3.2. Electroactive Properties of EPI-5 and Series of Au/EPI-5 Composites by Electrochemical CV Studies
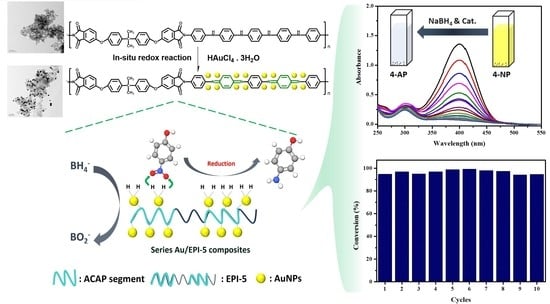
3.4. Catalytic Characterization of EPI-5 and Series of Au/EPI-5 Composites
3.5. Possible Reduction Reaction Mechanism for 4-NP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Shi, X.; Hua, R.; Zhang, R.; Yao, Y.; Zhao, B.; Liu, T.; Zheng, J.; Lu, G. Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@ COF supported noble metal nanoparticles. Appl. Catal. B Environ. 2020, 260, 118142. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. High performance and ultrafast reduction of 4-nitrophenol using metal-organic frameworks. J. Environ. Chem. Eng. 2021, 9, 104404. [Google Scholar] [CrossRef]
- Hauchman, F.S. US Environmental Protection Agency Health-Effects Research on Drinking-Water Contaminants; Environmental Protection Agency: Research Triangle Park, NC, USA, 1992.
- Liu, S.; Wang, J.; Huang, W.; Tan, X.; Dong, H.; Goodman, B.A.; Du, H.; Lei, F.; Diao, K. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2019, 214, 821–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Liu, X.; Yin, Z.; Cao, S. Silver/carbon co-decorated hollow TiO2 catalyst drives the efficient photocatalytic degradation/catalytic hydrogenation of 4-nitrophenol. J. Taiwan Inst. Chem. Eng. 2022, 133, 104274. [Google Scholar] [CrossRef]
- Sarkar, P.; Dey, A. 4-Nitrophenol biodegradation by an isolated and characterized microbial consortium and statistical optimization of physicochemical parameters by Taguchi Methodology. J. Environ. Chem. Eng. 2020, 8, 104347. [Google Scholar] [CrossRef]
- Menazea, A.; Mostafa, A.M. Ag doped CuO thin film prepared via pulsed laser deposition for 4-nitrophenol degradation. J. Environ. Chem. Eng. 2020, 8, 104104. [Google Scholar] [CrossRef]
- Chu, Y.; Miao, B.; Zhang, X.; Lv, R. Heterogeneous electro-Fenton-like oxidation for the degradation of 4-nitrophenol characterized by immobilized Fe (III): Performance, mechanism and chlorinated organic compounds formation. J. Water Process Eng. 2020, 38, 101662. [Google Scholar] [CrossRef]
- Hu, L.; Liu, X.; Guo, A.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Cobalt with porous carbon architecture: Towards of 4-nitrophenol degradation and reduction. Sep. Purif. Technol. 2022, 288, 120595. [Google Scholar] [CrossRef]
- Liang, W.; Lu, Y.; Li, N.; Li, H.; Zhu, F. Microwave-assisted synthesis of magnetic surface molecular imprinted polymer for adsorption and solid phase extraction of 4-nitrophenol in wastewater. Microchem. J. 2020, 159, 105316. [Google Scholar] [CrossRef]
- Mejía, Y.R.; Bogireddy, N.K.R. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.F. An historical review of the use of dye precursors in the formulation of commercial oxidation hair dyes. Dye Pigment. 1999, 41, 127–136. [Google Scholar] [CrossRef]
- Lunar, L.; Sicilia, D.; Rubio, S.; Pérez-Bendito, D.; Nickel, U. Degradation of photographic developers by Fenton’s reagent: Condition optimization and kinetics for metol oxidation. Water Res. 2000, 34, 1791–1802. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, D.-H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664–669. [Google Scholar] [CrossRef]
- Hao, B.; Lu, G.; Zhang, S.; Li, Y.; Ding, A.; Huang, X. Gold nanoparticles standing on PEG/PAMAM/thiol-functionalized nanographene oxide as aqueous catalysts. Polym. Chem. 2020, 11, 4094–4104. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Almuqati, T.; Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes. J. Clean. Prod. 2018, 191, 429–435. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: A review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Futamura, S.; Muramoto, A.; Tachikawa, Y.; Matsuda, J.; Lyth, S.M.; Shiratori, Y.; Taniguchi, S.; Sasaki, K. SOFC anodes impregnated with noble metal catalyst nanoparticles for high fuel utilization. Int. J. Hydrogen Energy 2019, 44, 8502–8518. [Google Scholar] [CrossRef]
- Liu, D.; Barbar, A.; Najam, T.; Javed, M.S.; Shen, J.; Tsiakaras, P.; Cai, X. Single noble metal atoms doped 2D materials for catalysis. Appl. Catal. B Environ. 2021, 297, 120389. [Google Scholar] [CrossRef]
- Das, R.; Sypu, V.S.; Paumo, H.K.; Bhaumik, M.; Maharaj, V.; Maity, A. Silver decorated magnetic nanocomposite (Fe3O4@ PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B Environ. 2019, 244, 546–558. [Google Scholar] [CrossRef]
- Lai, G.-H.; Huang, T.-C.; Pai, Y.-H.; Huang, B.-S.; Tsai, M.-H.; Yang, T.-I.; Chung, Y.-H. Preparation of highly-stable and recyclable novel Au/ZrP composite catalyst for 4-nitrophenol reduction. J. Taiwan Inst. Chem. Eng. 2019, 95, 525–531. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, S.K.; Das, N.C. Green synthesis of a reduced graphene oxide/silver nanoparticles-based catalyst for degradation of a wide range of organic pollutants. Nano-Struct. Nano-Objects 2023, 34, 100960. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, J.; Wen, W.; Zhang, X.; Wang, S. Spherical covalent organic framework supported Cu/Ag bimetallic nanoparticles with highly catalytic activity for reduction of 4-nitrophenol. J. Solid State Chem. 2022, 311, 123116. [Google Scholar] [CrossRef]
- Khan, A.; Wei, D.; Wang, Z.; Su, X.; Wang, J.; Alam, S.; Wang, L.; Wu, R.; Maloletnev, A.S.; Yang, C. MOF-derived nickel–cobalt bimetal oxide nanostructures as a cooperative catalyst for the reduction of 4-nitrophenol. J. Chem. Technol. Biotechnol. 2021, 96, 697–703. [Google Scholar] [CrossRef]
- Wang, M.; Tang, X.-H.; Cai, J.-H.; Wu, H.; Shen, J.-B.; Guo, S.-Y. Construction, mechanism and prospective of conductive polymer composites with multiple interfaces for electromagnetic interference shielding: A review. Carbon 2021, 177, 377–402. [Google Scholar] [CrossRef]
- Sun, L.; Yin, Z.; Zhang, J.; Ren, X.; Zhang, M.; Song, W.; Xu, Z.; Qi, C. Gold nanoparticles supported on poly (aniline-co-pyrrole) as the efficient catalysts for the reduction of 4-nitrophenol. Mol. Catal. 2022, 525, 112362. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Cui, M.; Wang, X.; Chee, K.J.; Nguyen, V.C.; Kumar, V.; Sumboja, A.; Wang, M.; Lee, P.S. Aniline Tetramer-Graphene Oxide Composites for High Performance Supercapacitors. Adv. Energy Mater. 2014, 4, 1400781. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Y.; Mao, H.; Lu, X.; Zhang, W.; Wei, Y. Synthesis of parent aniline tetramer and pentamer and redox properties. Mater. Lett. 2005, 59, 2446–2450. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, H.; Qi, F.; Dong, Z.; Mu, J.; Qiu, X. Synthesis and electrochemical properties of polyimide containing nona-aniline. React. Funct. Polym. 2022, 178, 105333. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Berda, E.B.; Zhao, J.; Liu, X.; Chao, D. Design and synthesis of multicolor electrochromic polymers based on oligoaniline and viologen/phenothiazine groups. Eur. Polym. J. 2020, 138, 109979. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Fang, W.; Zhao, Q.; Lei, X.; Zhang, J.; Chen, J.; Li, Y.; Zuo, Y. Synergetic Effect of Electrical and Topographical Cues in Aniline Trimer-Based Polyurethane Fibrous Scaffolds on Tissue Regeneration. J. Funct. Biomater. 2023, 14, 185. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, L.-C.; Lai, G.-H.; Huang, B.-S.; Yang, T.-I.; Hsu, S.-C.; Lo, A.-Y.; Yeh, J.-M. Advanced superhydrophobic electroactive fluorinated polyimide and its application in anticorrosion coating. Int. J. Green Energy 2017, 14, 113–120. [Google Scholar] [CrossRef]
- Ji, W.-F.; Chu, C.-M.; Hsu, S.-C.; Lu, Y.-D.; Yu, Y.-C.; Santiago, K.S.; Yeh, J.-M. Synthesis and characterization of organo-soluble aniline oligomer-based electroactive doped with gold nanoparticles, and application to electrochemical sensing of ascorbic acid. Polymer 2017, 128, 218–228. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, H.J.; Huang, K.Y.; Yeh, J.M.; Liou, G.S. Novel organosoluble aromatic polyimides bearing pendant methoxy-substituted triphenylamine moieties: Synthesis, electrochromic, and gas separation properties. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7937–7949. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, T.-C.; Huang, H.-Y.; Ji, W.-F.; Chou, Y.-C.; Hung, W.-I.; Yeh, J.-M.; Tsai, M.-H. Electrochemical studies on aniline-pentamer-based electroactive polyimide coating: Corrosion protection and electrochromic properties. Electrochim. Acta 2011, 56, 10151–10158. [Google Scholar] [CrossRef]
- Chen, H.; Zhuang, Q.; Wang, H.; Zhai, X.; Zhang, K.; Deng, H.; Dong, W.; Xie, A. Ultrafine gold nanoparticles dispersed in conjugated microporous polymers with sulfhydryl functional groups to improve the reducing activity of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129459. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, L.; Peng, S.; Zheng, Y.; Sun, X.; Su, H.; Qi, C. Preparation of Au catalysts supported on core-shell SiO2/polypyrrole composites with high catalytic performances in the reduction of 4-nitrophenol. Synth. Met. 2019, 248, 20–26. [Google Scholar] [CrossRef]
- Lai, G.-H.; Huang, B.-S.; Yang, T.-I.; Tsai, M.-H.; Chou, Y.-C. Preparation of highly stable and recyclable Au/electroactive polyamide composite catalyst for nitrophenol reduction. Polymer 2021, 213, 123200. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A novel application of electroactive polyimide doped with gold nanoparticles: As a chemiresistor sensor for hydrogen sulfide gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.-X.; Weng, C.W.; Ahmed, M.M.; Luo, K.-H.; Ji, W.-F.; Liu, W.-R.; Yeh, J.-M. Aniline pentamer-modified reduced graphene oxide/epoxy composites as anticorrosion coatings. Mater. Chem. Phys. 2021, 264, 124446. [Google Scholar] [CrossRef]
- Tsai, M.; Lu, S.; Lai, Y.; Lai, G.; Dizon, G.; Yang, T.; Lin, Y.; Chou, Y. Novel ascorbic acid sensor prepared from gold/aniline-pentamer-based electroactive polyamide composites. Express Polym. Lett. 2018, 12, 71–81. [Google Scholar] [CrossRef]
- Yeh, L.-C.; Huang, T.-C.; Huang, Y.-P.; Huang, H.-Y.; Chen, H.-H.; Yang, T.-I.; Yeh, J.-M. Synthesis electroactive polyurea with aniline-pentamer-based in the main chain and its application in electrochemical sensor. Electrochim. Acta 2013, 94, 300–306. [Google Scholar] [CrossRef]
- Yan, Y.; Li, F.; Hanlon, A.M.; Berda, E.B.; Liu, X.; Wang, C.; Chao, D. Electrochemical performance of electroactive poly (amic acid)-Cu2+ composites. Appl. Surf. Sci. 2017, 392, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Liu, X.; Liu, W.; Gu, H. ROMP synthesis of gallol-containing polymer hydrogels for in situ fabrication of AuNPs and AgNPs composites as recyclable catalysts for the degradation of 4-nitrophenol. Polymer 2021, 219, 123539. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, L.; Zhang, J.; Murayama, T.; Zhang, M.; Zheng, Y.; Su, H.; Qi, C. Preparation of polyaniline microtubes as the gold catalyst support with improved catalytic performances for the reduction of nitrophenols. Top. Catal. 2021, 64, 215–223. [Google Scholar] [CrossRef]
- Shi, B.; Li, H.; Fu, X.; Zhao, C.; Li, M.; Liu, M.; Yan, W.; Yang, H. Fe Single-Atom Catalyst for Cost-Effective yet Highly Efficient Heterogeneous Fenton Catalysis. ACS Appl. Mater. Interfaces 2022, 14, 53767–53776. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Lee, S.W.; Moon, K.H.; Cho, S. Fabrication of solid-state asymmetric supercapacitors based on aniline oligomers and graphene electrodes with enhanced electrochemical performances. ACS Omega 2019, 4, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-J.; Hsu, P.-H.; Hsu, S.-C.; Chang, C.-H.; Hung, W.-I.; Wu, P.-S.; Yeh, J.-M. Synthesis of electroactive mesoporous gold–organosilica nanocomposite materials via a sol–gel process with non-surfactant templates and the electroanalysis of ascorbic acid. J. Mater. Chem. B 2013, 1, 4983–4991. [Google Scholar] [CrossRef]
- Latif, H.; Wasif, D.; Rasheed, S.; Sattar, A.; Rafique, M.S.; Anwar, A.W.; Zaheer, S.; Shabbir, S.A.; Imtiaz, A.; Qutab, M. Gold nanoparticles mixed multiwall carbon nanotubes, supported on graphene nano-ribbons (Au-NT-G) as an efficient reduction electrode for Polymer Electrolyte Membrane fuel cells (PEMFC). Renew. Energy 2020, 154, 767–773. [Google Scholar] [CrossRef]
- Balamurugan, A.; Ho, K.-C.; Chen, S.-M. One-pot synthesis of highly stable silver nanoparticles-conducting polymer nanocomposite and its catalytic application. Synth. Met. 2009, 159, 2544–2549. [Google Scholar] [CrossRef]
- Lai, G.-H.; Chou, Y.-C.; Huang, B.-S.; Yang, T.-I.; Tsai, M.-H. Application of electroactive Au/aniline tetramer–graphene oxide composites as a highly efficient reusable catalyst. RSC Adv. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Song, S.G.; Satheeshkumar, C.; Park, J.; Ahn, J.; Premkumar, T.; Lee, Y.; Song, C. N-heterocyclic carbene-based conducting polymer–gold nanoparticle hybrids and their catalytic application. Macromolecules 2014, 47, 6566–6571. [Google Scholar] [CrossRef]
- Lai, G.-H.; Huang, T.-C.; Huang, B.-S.; Chou, Y.-C. A novel Au/electroactive poly (amic acid) composite as an effective catalyst for p-nitrophenol reduction. RSC Adv. 2021, 11, 33990–33995. [Google Scholar] [CrossRef]
- Kumar, A.; Belwal, M.; Maurya, R.R.; Mohan, V.; Vishwanathan, V. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by Au/AC nanocatalysts. Mater. Sci. Energy Technol. 2019, 2, 526–531. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Gholami, M.R. Fabrication of novel ternary Au/CeO2@gC3N4 nanocomposite: Kinetics and mechanism investigation of 4-nitrophenol reduction, and benzyl alcohol oxidation. Appl. Phys. A 2018, 124, 1–17. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Q.; Xi, K. Synthesis of melamine-based crystalline porous polymers and its silver-doped composites with one-pot approach for catalytic reduction of 4-nitrophenol. Microporous Mesoporous Mater. 2022, 346, 112297. [Google Scholar] [CrossRef]
- Ma, J.; Deng, H.; Zhang, Z.; Zhang, L.; Qin, Z.; Zhang, Y.; Gao, L.; Jiao, T. Facile synthesis of Ag3PO4/PPy/PANI ternary composites for efficient catalytic reduction of 4-nitrophenol and 2-nitroaniline. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127774. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Du, F.; Wu, Y.; Zhang, Q.; Jiang, C. Silver nanoparticles/polydopamine coated polyvinyl alcohol sponge as an effective and recyclable catalyst for reduction of 4-nitrophenol. Mater. Chem. Phys. 2019, 225, 42–49. [Google Scholar] [CrossRef]
- Sypu, V.S.; Bhaumik, M.; Raju, K.; Maity, A. Nickel hydroxide nanoparticles decorated napthalene sulfonic acid-doped polyaniline nanotubes as efficient catalysts for nitroarene reduction. J. Colloid Interface Sci. 2021, 581, 979–989. [Google Scholar] [CrossRef]
- Dey, C.; De, D.; Nandi, M.; Goswami, M.M. A high performance recyclable magnetic CuFe2O4 nanocatalyst for facile reduction of 4-nitrophenol. Mater. Chem. Phys. 2020, 242, 122237. [Google Scholar] [CrossRef]


















| Benzene Ring (1500 cm−1) | Quinone Ring (1595 cm−1) | B/Q | Loading of Au (wt.%) a | |
|---|---|---|---|---|
| EPI-5 | 0.1829 | 0.0806 | 2.27 | 0 |
| 1Au/EPI-5 | 0.1423 | 0.0818 | 1.74 | 4.73 |
| 3Au/EPI-5 | 0.1908 | 0.1112 | 1.71 | 10.86 |
| 5Au/EPI-5 | 0.1526 | 0.0932 | 1.63 | 17.46 |
| Catalysts | Binding Energies (eV) | N Species (%) | ||||
|---|---|---|---|---|---|---|
| -NH- | -N= | N+ | -NH- | -N= | N+ | |
| EPI-5 | 397.4 | 398.9 | 402.0 | 63.9 | 28.2 | 7.9 |
| 5Au/EPI-5 | 397.1 | 398.9 | 401.4 | 26.7 | 65.2 | 8.1 |
| Catalyst | Weight (mg) | Rate Constant (s−1) | Rate Constant (min−1) | Recyclability (Cycles) | Ref. |
|---|---|---|---|---|---|
| AuNPs-gel-G | 20 | - | 0.081 | 5 | [46] |
| ME-BTCA-Ag-2 | 5 | 3.3 × 10−4 | - | - | [57] |
| Ag3PO4/PPy/PANI | 1 | - | 0.005 | - | [58] |
| AgNPs/D-PVA | 60 | - | 0.072 | 4 | [59] |
| Fe3O4@PPy-MAA-Ag | 2.5 | - | 0.005 | 8 | [21] |
| Au/ZrP | 1 | 1.8 × 10−2 | - | 10 | [22] |
| Au/EPA | 0.5 | 1.6 × 10−2 | - | 30 | [39] |
| 5Au/EPI-5 | 0.5 | 1.1 × 10−3 | 0.064 | 10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Shi, W.-Z.; Luo, K.-H.; Yeh, J.-M.; Tsai, M.-H. In Situ Redox Synthesis of Highly Stable Au/Electroactive Polyimide Composite and Its Application on 4-Nitrophenol Reduction. Polymers 2023, 15, 2664. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122664
Chen Y-S, Shi W-Z, Luo K-H, Yeh J-M, Tsai M-H. In Situ Redox Synthesis of Highly Stable Au/Electroactive Polyimide Composite and Its Application on 4-Nitrophenol Reduction. Polymers. 2023; 15(12):2664. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122664
Chicago/Turabian StyleChen, Yi-Sheng, Wei-Zhong Shi, Kun-Hao Luo, Jui-Ming Yeh, and Mei-Hui Tsai. 2023. "In Situ Redox Synthesis of Highly Stable Au/Electroactive Polyimide Composite and Its Application on 4-Nitrophenol Reduction" Polymers 15, no. 12: 2664. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122664