Synergistic Effects and Mechanistic Insights into the Co-Hydropyrolysis of Chilean Oak and Polyethylene: Unlocking the Potential of Biomass–Plastic Valorisation
Abstract
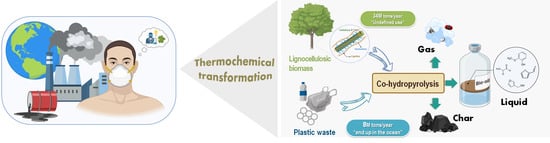
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Experimental Setup
2.3. Thermogravimetric Analysis
2.4. Experimental Design
2.5. Synergy Coefficient Analysis
3. Results
3.1. Thermogravimetric Analysis
3.2. Experimental Design Analysis
3.3. Synergistic Effect
3.4. Reaction Mechanism Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roser, M. Global Direct Primary Energy Consumption. Available online: https://ourworldindata.org/grapher/global-primary-energy (accessed on 18 June 2023).
- Huntington, T.; Cui, X.; Mishra, U.; Scown, C.D. Machine Learning to Predict Biomass Sorghum Yields under Future Climate Scenarios. Biofuels Bioprod. Biorefining 2020, 14, 566–577. [Google Scholar] [CrossRef]
- Asif, M.; Muneer, T. Energy Supply, Its Demand and Security Issues for Developed and Emerging Economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Sandberg, E.; Krook-Riekkola, A. The Impact of Technology Availability on the Transition to Net-Zero Industry in Sweden. J. Clean. Prod. 2022, 363, 132594. [Google Scholar] [CrossRef]
- Maqhuzu, A.B.; Yoshikawa, K.; Takahashi, F. The Effect of Coal Alternative Fuel from Municipal Solid Wastes Employing Hydrothermal Carbonization on Atmospheric Pollutant Emissions in Zimbabwe. Sci. Total Environ. 2019, 668, 743–759. [Google Scholar] [CrossRef]
- Velazquez, H.D.; Likhanova, N.; Aljammal, N.; Verpoort, F.; Martínez-Palou, R. New Insights into the Progress on the Isobutane/Butene Alkylation Reaction and Related Processes for High-Quality Fuel Production. A Critical Review. Energy Fuels 2020, 34, 15525–15556. [Google Scholar] [CrossRef]
- Gin, A.W.; Hassan, H.; Ahmad, M.A.; Hameed, B.H.; Mohd Din, A.T. Recent Progress on Catalytic Co-Pyrolysis of Plastic Waste and Lignocellulosic Biomass to Liquid Fuel: The Influence of Technical and Reaction Kinetic Parameters. Arab. J. Chem. 2021, 14. [Google Scholar] [CrossRef]
- Carrasco, S.; Silva, J.; Pino-Cortés, E.; Gómez, J.; Vallejo, F.; Díaz-Robles, L.; Campos, V.; Cubillos, F.; Pelz, S.; Paczkowski, S.; et al. Experimental Study on Hydrothermal Carbonization of Lignocellulosic Biomass with Magnesium Chloride for Solid Fuel Production. Processes 2020, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, F.; Diaz-Robles, L.A.; Gonzalez, P.; Poblete, J. Energy efficiency evaluation of a continuous treatment of agroforestry waste biomass by hydrothermal carbonisation. Maderas Cienc. Y Tecnol. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sahoo, A.; Mohanty, K. Pyrolysis Kinetics and Synergistic Effect in Co-Pyrolysis of Samanea Saman Seeds and Polyethylene Terephthalate Using Thermogravimetric Analyser. Bioresour. Technol. 2019, 289, 121608. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, Xylan and Lignin Interactions during Pyrolysis of Lignocellulosic Biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. A Comparative Study on Co-Pyrolysis of Lignocellulosic Biomass with Polyethylene Terephthalate, Polystyrene, and Polyvinyl Chloride: Synergistic Effects and Product Characteristics. J. Clean. Prod. 2018, 205, 1127–1138. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Thermo-Catalytic Co-Pyrolysis of Waste Plastic and Paper in Batch and Tubular Reactors for in-Situ Product Improvement. J. Environ. Manag. 2020, 269, 110741. [Google Scholar] [CrossRef]
- Dabros, T.M.H.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation Fuels from Biomass Fast Pyrolysis, Catalytic Hydrodeoxygenation, and Catalytic Fast Hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-Pyrolysis of Waste Plastic and Solid Biomass for Synergistic Production of Biofuels and Chemicals-A Review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Cao, B.; Sun, Y.; Guo, J.; Wang, S.; Yuan, J.; Esakkimuthu, S.; Bernard Uzoejinwa, B.; Yuan, C.; Abomohra, A.E.F.; Qian, L.; et al. Synergistic Effects of Co-Pyrolysis of Macroalgae and Polyvinyl Chloride on Bio-Oil/Bio-Char Properties and Transferring Regularity of Chlorine. Fuel 2019, 246, 319–329. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Gebreegziabher, T.; Ng, D.K.S.; Hui, C.W. Mixed-Waste Pyrolysis of Biomass and Plastics Waste—A Modelling Approach to Reduce Energy Usage. Energy 2014, 75, 127–135. [Google Scholar] [CrossRef]
- Yang, J.; Rizkiana, J.; Widayatno, W.B.; Karnjanakom, S.; Kaewpanha, M.; Hao, X.; Abudula, A.; Guan, G. Fast Co-Pyrolysis of Low Density Polyethylene and Biomass Residue for Oil Production. Energy Convers. Manag. 2016, 120, 422–429. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the Thermal Behavior, Kinetics, and Product Characterisation of Biomass and Low-Density Polyethylene Co-Pyrolysis by Thermogravimetric Analysis and Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Minitab, L. Minitab. 2021. [Google Scholar]
- Ahmad, M.S.; Liu, C.G.; Nawaz, M.; Tawab, A.; Shen, X.; Shen, B.; Mehmood, M.A. Elucidating the Pyrolysis Reaction Mechanism of Calotropis Procera and Analysis of Pyrolysis Products to Evaluate Its Potential for Bioenergy and Chemicals. Bioresour. Technol. 2021, 322, 124545. [Google Scholar] [CrossRef]
- Dai, G.; Wang, K.; Wang, G.; Wang, S. Initial Pyrolysis Mechanism of Cellulose Revealed by In-Situ DRIFT Analysis and Theoretical Calculation. Combust. Flame 2019, 208, 273–280. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, S.; Cong, K.; Li, Q.; Zhang, Y. Insight into Synergistic Effects of Biomass-Polypropylene Co-Pyrolysis Using Representative Biomass Constituents. Bioresour. Technol. 2020, 307, 123243. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y.; et al. Bio-Oil from Fast Pyrolysis of Lignin: Effects of Process and Upgrading Parameters. Bioresour. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef]
- Alejandro-Martín, S.; Cerda-Barrera, C.; Montecinos, A. Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite. Catalysts 2017, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wang, Y.; Huang, Q.; Cai, J. Thermogravimetric Characteristics and Kinetic of Plastic and Biomass Blends Co-Pyrolysis. Fuel Process. Technol. 2006, 87, 963–969. [Google Scholar] [CrossRef]
- Wen, Y.; Zaini, I.N.; Wang, S.; Mu, W.; Jönsson, P.G.; Yang, W. Synergistic Effect of the Co-Pyrolysis of Cardboard and Polyethylene: A Kinetic and Thermodynamic Study. Energy 2021, 229, 120693. [Google Scholar] [CrossRef]
- Mohabeer, C.; Reyes, L.; Abdelouahed, L.; Marcotte, S.; Taouk, B. Investigating Catalytic De-Oxygenation of Cellulose, Xylan and Lignin Bio-Oils Using HZSM-5 and Fe-HZSM-5. J. Anal. Appl. Pyrolysis 2019, 137, 118–127. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Yu, T.; Cai, Q. Synergistic Effect of Co-Pyrolysis of Biomass and Plastics. Chem. Ind. For. Prod. 2019, 39, 34–42. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-Pyrolysis of Biomass and Waste Plastics as a Thermochemical Conversion Technology for High-Grade Biofuel Production: Recent Progress and Future Directions Elsewhere Worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Mullen, C.A.; Dorado, C.; Boateng, A.A. Catalytic Co-Pyrolysis of Switchgrass and Polyethylene over HZSM-5: Catalyst Deactivation and Coke Formation. J. Anal. Appl. Pyrolysis 2018, 129, 195–203. [Google Scholar] [CrossRef]
- Oladunni, A.A.; Odejobi, O.J.; Sanda, O.; Sonibare, J.A. Optimization of Combined Mixture and Process Variables for the Pyrolysis Oil Yield from Co-Pyrolysis of Polymeric Wastes. Environ. Qual. Manag. 2021, 30, 83–99. [Google Scholar] [CrossRef]
- Kayacan, I.; Doǧan, Ö.M. Pyrolysis of Low and High Density Polyethylene. Part II: Analysis of Liquid Products Using FTIR and NMR Spectroscopy. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 392–400. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Fathi, M.; Bazgir, H.; Ghadiri, M. The Pyrolysis of Oak with Polyethylene, Polypropylene and Polystyrene Using Fixed Bed and Stirred Reactors and TGA Instrument. Energy 2021, 232, 121085. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, S.; Brown, R.C.; Kelkar, A.; Bai, X. Fast Pyrolysis of Biomass and Waste Plastic in a Fluidized Bed Reactor. Fuel 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Catalytic Co-Pyrolysis of Sugarcane Bagasse and Waste High-Density Polyethylene over Faujasite-Type Zeolite. Bioresour. Technol. 2019, 284, 406–414. [Google Scholar] [CrossRef]
- Chen, W.; Shi, S.; Chen, M.; Zhou, X. Fast Co-Pyrolysis of Waste Newspaper with High-Density Polyethylene for High Yields of Alcohols and Hydrocarbons. Waste Manag. 2017, 67, 155–162. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B.H.; Lim, J.K. Co-Pyrolysis of Sugarcane Bagasse and Waste High-Density Polyethylene: Synergistic Effect and Product Distributions. Energy 2020, 191, 116545. [Google Scholar] [CrossRef]
- Chen, W.; Shi, S.; Zhang, J.; Chen, M.; Zhou, X. Co-Pyrolysis of Waste Newspaper with High-Density Polyethylene: Synergistic Effect and Oil Characterization. Energy Convers. Manag. 2016, 112, 41–48. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.; Lam, S.S.; Kwon, E.E.; Ha, J.M.; Tsang, D.C.W.; Ok, Y.S.; Chen, W.H.; Park, Y.K. Fast Hydropyrolysis of Biomass Conversion: A Comparative Review. Bioresour. Technol. 2021, 342. [Google Scholar] [CrossRef]
- Alejandro-Martín, S.; Acaricia, A.M.; Cerda-Barrera, C.; Pérez, H.D. Influence of Chemical Surface Characteristics of Ammonium-Modified Chilean Zeolite on Oak Catalytic Pyrolysis. Catalysts 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, Pyrolysis and Two-Stage Thermodegradation of Hemicellulose, Cellulose and Lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic Co-Pyrolysis of Lignocellulosic Biomass with Polymers: A Critical Review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, C.; Xie, Y.; Wang, Y.; Wang, J.; Zhang, R. Aromatic Hydrocarbons Production and Synergistic Effect of Plastics and Biomass via One-Pot Catalytic Co-Hydropyrolysis on HZSM-5. J. Anal. Appl. Pyrolysis 2020, 147. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. Thermal Behavior and Kinetic Study for Catalytic Co-Pyrolysis of Biomass with Plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed Kinetic Modeling of the Thermal Degradation of Lignins. Biomass Bioenergy 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Li, R.; Wu, Y.; Yang, M. A Modified Kinetic Analysis Method of Cellulose Pyrolysis Based on TG–FTIR Technique. Thermochim. Acta 2018, 665, 20–27. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Beregovtsova, N.G.; Kuznetsov, B.N.; Membrado, L.; Cebolla, V.L.; Marin, N.; Weber, J.V. Co-Pyrolysis of Wood Biomass and Synthetic Polymers Mixtures. Part III: Characterisation of Heavy Products. J. Anal. Appl. Pyrolysis 2003, 2, 325–340. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic Effects of Catalytic Co-Pyrolysis of Macroalgae with Waste Plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Evolution of Products Generated during the Dynamic Pyrolysis of LDPE and HDPE over HZSM5. Energy Fuels 2008, 22, 2917–2924. [Google Scholar] [CrossRef]
- Yuan, H.; Fan, H.; Shan, R.; He, M.; Gu, J.; Chen, Y. Study of Synergistic Effects during Co-Pyrolysis of Cellulose and High-Density Polyethylene at Various Ratios. Energy Convers. Manag. 2018, 157, 517–526. [Google Scholar] [CrossRef]






| Operational Parameter | Levels | DoE Coded Variables |
|---|---|---|
| Plastic type | ChO:LDPE | −1 |
| ChO:HDPE | +1 | |
| ChO:plastics | 1:0 | |
| 2:1 | −1 | |
| 1:1 | 0 | |
| 1:2 | +1 | |
| 0:1 | ||
| Hydrogen pressure (psi) | 100 | −1 |
| 150 | 0 | |
| 200 | +1 | |
| Reactor heating rate (°C/s) | 12.5 | −1 |
| 25.0 | ||
| 10,000 | +1 |
| Compound Group | A | B | C | D | AC | AD | BC | BD | CC | ACC |
|---|---|---|---|---|---|---|---|---|---|---|
| Acids | ++ | + | ||||||||
| Acetic acid | ++ | ++ | − | |||||||
| Propanoic acid, 2-oxo-, methyl ester | + | −− | + | − | ||||||
| Acetic anhydride | ++ | ++ | ++ | |||||||
| Alcohols | −− | −− | ++ | ++ | ||||||
| 1,2-Ethanediol, monoacetate | + | −− | ||||||||
| 2-Furanmethanol | ++ | ++ | ||||||||
| Aldehydes | ++ | ++ | ||||||||
| Furfural | ++ | ++ | − | |||||||
| Succindialdehyde | ++ | ++ | + | + | ||||||
| Hydrocarbons | ++ | + | − | |||||||
| 1-Pentene, 4-methyl | ++ | ++ | ++ | ++ | ||||||
| 1-Tridecene | − | |||||||||
| 1-Nonene | ++ | − | − | |||||||
| 1-Octene,3,7, dimethyl | ++ | − | ||||||||
| Ketones | ++ | + | − | |||||||
| 2-Propanone, 1-hydroxy- | ++ | ++ | − | |||||||
| 3-Methylcyclopentane-1,2-dione | ++ | + | ||||||||
| Phenols | ++ | −− | − | |||||||
| Phenol, 2,6-dimethoxy- | ++ | −− | − | |||||||
| Creosol | ++ | + | − | |||||||
| Phenol, 2-methoxy- | ++ | − | ++ | − | ||||||
| 2-Methoxy-4-vinyl phenol | ++ | −− | ++ | |||||||
| Oxygenated compounds | ++ | + | −− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puentes, B.; Vallejo, F.; Alejandro-Martín, S. Synergistic Effects and Mechanistic Insights into the Co-Hydropyrolysis of Chilean Oak and Polyethylene: Unlocking the Potential of Biomass–Plastic Valorisation. Polymers 2023, 15, 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122747
Puentes B, Vallejo F, Alejandro-Martín S. Synergistic Effects and Mechanistic Insights into the Co-Hydropyrolysis of Chilean Oak and Polyethylene: Unlocking the Potential of Biomass–Plastic Valorisation. Polymers. 2023; 15(12):2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122747
Chicago/Turabian StylePuentes, Bastián, Fidel Vallejo, and Serguei Alejandro-Martín. 2023. "Synergistic Effects and Mechanistic Insights into the Co-Hydropyrolysis of Chilean Oak and Polyethylene: Unlocking the Potential of Biomass–Plastic Valorisation" Polymers 15, no. 12: 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15122747