Formation Features of Polymer–Metal–Carbon Ternary Electromagnetic Nanocomposites Based on Polyphenoxazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
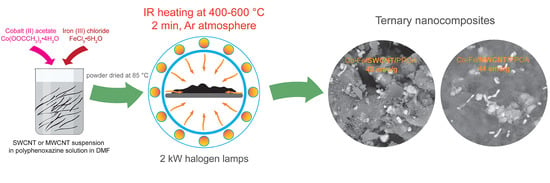
2.2. Synthesis of Co-Fe/CNT/PPOA
2.3. Characterization
3. Results
3.1. Characterization of Co-Fe/CNT/PPOA Nanomaterials
3.2. Electromagnetic Properties of Co-Fe/CNT/PPOA Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Sun, Q.; Li, J.; Guo, Y.; Tian, W.; Liu, Y.; Wu, B.; Deng, L.; Mahmood, N.; Jian, X. Polymer-based nanocomposites: Role of interface for effective microwave absorption. Mater. Today Phys. 2023, 31, 100981. [Google Scholar] [CrossRef]
- Maleki, S.T.; Babamoradi, M. Microwave absorption theory and recent advances in microwave absorbers by polymer-based nanocomposites (carbons, oxides, sulfides, metals, and alloys). Inorg. Chem. Commun. 2023, 149, 110407. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N. Conducting polymer based composites as efficient EMI shielding materials: A comprehensive review and future prospects. Synth. Met. 2021, 272, 116664. [Google Scholar] [CrossRef]
- Goswami, S.; Nandy, S.; Fortunato, E.; Martins, R. Polyaniline and its composites engineering: A class of multifunctional smart energy materials. J. Solid State Chem. 2023, 317, 123679. [Google Scholar] [CrossRef]
- Yang, R.B.; Reddy, P.M.; Chang, C.J.; Chen, P.A.; Chen, J.K.; Chang, C.C. Synthesis and characterization of Fe3O4/polypyrrole/carbon nanotube composites with tunable microwave absorption properties: Role of carbon nanotube and polypyrrole content. Chem. Eng. J. 2016, 285, 497–507. [Google Scholar] [CrossRef]
- Sedighi, A.; Naderi, M.; Brycki, B. Wearable nonwoven fabric decorated with Fe3O4/rGO/PANI/Ni-P for efficient electromagnetic interference shielding. J. Alloys Compd. 2023, 938, 168454. [Google Scholar] [CrossRef]
- Idumah, C.I. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth. Met. 2021, 273, 116674. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Sun, Y.; Chen, H.; Xiong, Z.; Li, X.; Shen, L.; Liu, Y. Tunable magnetic properties of Fe3O4/rGO/PANI nanocomposites for enhancing microwave absorption performance. J. Alloys Compd. 2019, 796, 120–130. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Zhao, X.; Gai, X.; An, W.; Fang, G.; Zhang, A.; Chen, X. Polypyrrole coated Fe3O4 nanoparticles decorated carbon nanotubes nanocomposites and the microwave absorption properties. J. Mater. Sci. Mater. Electron. 2019, 30, 17333–17341. [Google Scholar] [CrossRef]
- Dalal, J.; Lather, S.; Gupta, A.; Tripathi, R.; Maan, A.S.; Singh, K.; Ohlan, A. Reduced graphene oxide functionalized strontium ferrite in poly(3,4-ethylenedioxythiophene) conducting network: A high-performance EMI shielding material. Adv. Mater. Technol. 2019, 4, 1900023. [Google Scholar] [CrossRef]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.-X.; Liu, T. A review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Chahal, P.; Madaswamy, S.L.; Lee, S.C.; Wabaidur, S.M.; Dhayalan, V.; Ponnusamy, V.K.; Dhanusuraman, R. Novel manganese oxide decorated polyaniline/graphitic carbon nitride nanohybrid material for efficient supercapacitor application. Fuel 2022, 330, 125531. [Google Scholar] [CrossRef]
- Lin, H.; Huang, Q.; Wang, J.; Jiang, J.; Liu, F.; Chen, Y.; Wang, C.; Lu, D.; Han, S. Self-assembled graphene/polyaniline/Co3O4 ternary hybrid aerogels for supercapacitors. Electrochim. Acta 2016, 191, 444–451. [Google Scholar] [CrossRef]
- Ghanbari, R.; Ghorbani, S.R. High-performance nickel molybdate/reduce graphene oxide/polypyrrole ternary nanocomposite as flexible all-solid-state asymmetric supercapacitor. J. Energy Storage 2023, 60, 106670. [Google Scholar] [CrossRef]
- Payami, E.; Teimuri-Mofrad, R. Ternary nanocomposite of GQDs-polyFc/Fe3O4/PANI: Design, synthesis, and applied for electrochemical energy storage. Electrochim. Acta 2023, 439, 141706. [Google Scholar] [CrossRef]
- Patil, V.S.; Thoravat, S.S.; Kundale, S.S.; Dongale, T.D.; Patil, P.S.; Jadhav, S.A. Synthesis and testing of polyaniline grafted functional magnetite (Fe3O4) nanoparticles and rGO based nanocomposites for supercapacitor application. Chem. Phys. Lett. 2023, 814, 140334. [Google Scholar] [CrossRef]
- Azman, N.H.N.; Mamat, M.S.; Lim, H.N.; Sulaiman, Y. High-performance symmetrical supercapacitor based on poly(3,4)-ethylenedioxythiophene/graphene oxide/iron oxide ternary composite. J. Mater. Sci. Mater. Electron. 2018, 29, 6916–6923. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Sliwak, A.; Miniach, E.; Gryglewicz, G. Polypyrrole/iron oxide/reduced graphene oxide ternary composite as a binderless electrode material with high cyclic stability for supercapacitors. Compos. B 2017, 109, 23–29. [Google Scholar] [CrossRef]
- Lei, C.; Wang, C.; Chen, W.; He, M.; Huang, B. Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: Removal efficacy and mechanisms. Sci. Total Environ. 2020, 733, 139316. [Google Scholar] [CrossRef]
- Khan, M.I.; Almesfer, M.K.; Elkhaleefa, A.; Shigidi, I.; Shamim, M.Z.; Ali, I.H.; Rehan, M. Conductive polymers and their nanocomposites as adsorbents in environmental applications. Polymers 2021, 13, 3810. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Harthy, E.A.; Al Angari, Y.M.; Salam, M.A.; Awad, A.; Al-Juaid, A.A.; Saeed, A. Synthesis, characterization and dye removal capability of conducting polypyrrole/Mn0.8Zn0.2Fe2O4/graphite oxide ternary composites. Catalysts 2022, 12, 1624. [Google Scholar] [CrossRef]
- Al-Hasnawy, Z.A.A.N.; Jasim, K.K.; Awad, M.A. Preparation and characterization of zinc ferrite-polyaniline-graphene oxide nanocomposite for removal of orange G and malachite green dye from aqueous solution: As a model of water treatment and health environment. HIV Nur. 2023, 23, 463–474. Available online: https://hivnursing.net/index.php/hiv/article/view/1114 (accessed on 1 December 2022).
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W.; et al. The state of the art of natural polymer functionalized Fe3O4 magnetic nanoparticle composites for drug delivery applications: A review. Gels 2023, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Shi, P.; Sun, S.; Rui, M. Construction of rGO/Fe3O4/PANI nanocomposites and its corrosion resistance mechanism in waterborne acrylate-amino coating. Prog. Org. Coat. 2019, 133, 117–124. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, F.; Li, W.; Yang, H.; Zhang, X.; Liu, Y.; Ma, J. A facile preparation of CoFe2O4 nanoparticles on polyaniline-functionalised carbon nanotubes as enhanced catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 4472–4478. [Google Scholar] [CrossRef]
- Ren, G.; Li, Y.; Guo, Z.; Xiao, G.; Zhu, Y.; Dai, L.; Jiang, L. A bio-inspired Co3O4-polypyrrole-graphene complex as an efficient oxygen reduction catalyst in one-step ball milling. Nano Res. 2015, 8, 3461–3471. [Google Scholar] [CrossRef]
- Zhao, C.; Jin, Y.; Du, X.; Du, W. In situ prepared amorphous FeCoO-polyaniline/multiwalled carbon nanotube nanohybrids as efficient oxygen evolution catalysts for rechargeable Zn-air batteries. J. Power. Sources 2018, 399, 337–342. [Google Scholar] [CrossRef]
- Hojati, S.F.; Amiri, A.; MoeiniEghbali, N.; Mohamadi, S. Polypyrrole/Fe3O4/CNT as a recyclable and highly efficient catalyst for one-pot three-component synthesis of pyran derivatives. Appl. Organometal. Chem. 2018, 32, E4235. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A promising sensing platform on ternary composite of polyaniline-Fe2O3-reduced graphene oxide for sensitive hydroquinone determination. Chem. Eng. J. 2015, 259, 594–602. [Google Scholar] [CrossRef]
- Shokry, A.; Khalil, M.M.A.; Ibrahim, H.; Soliman, M.; Ebrahim, S. Highly luminescent ternary nanocomposite of polyaniline, silver nanoparticles and graphene oxide quantum dots. Sci. Rep. 2019, 9, 16984. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kwon, O.S.; Jang, J. A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chem. Commun. 2013, 49, 4673–4675. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yue, R.; Xu, J.; Liu, Z. One-pot synthesis of Fe2O3/PEDOT/rGO nanocomposite for sensitive determination of caffeine. Int. J. Electrochem. Sci. 2018, 13, 6791–6802. [Google Scholar] [CrossRef]
- Singh, A.P.; Mishra, M.; Sambyal, P.; Gupta, B.K.; Singh, B.P.; Chandra, A.; Dhawan, S.K. Encapsulation of γ-Fe2O3 decorated reduced graphene oxide in polyaniline core–shell tubes as an exceptional tracker for electromagnetic environmental pollution. J. Mater. Chem. A 2014, 2, 3581–3593. [Google Scholar] [CrossRef]
- Murugesan, B.; Pandiyan, N.; Sonamuthu, J.; Samayanan, S.; Mahalingam, S. Ternary nanocomposite designed by MWCNT backbone PPy/Pd for efficient catalytic approach toward reduction and oxidation reactions. Adv. Powder Technol. 2018, 29, 3173–3182. [Google Scholar] [CrossRef]
- Swathy, T.S.; Antony, M.J.; George, N. Active solvent hydrogen-enhanced p-nitrophenol reduction using heterogeneous silver nanocatalysts@surface-functionalized multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2021, 60, 7050–7064. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Kostev, A.I.; Karpacheva, G.P. Multifunctional nanocomposites based on polydiphenylamine-2-carboxylic acid, magnetite nanoparticles and single-walled carbon nanotubes. Polym. Bull. 2022, 79, 3721–3739. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Petrov, V.A.; Chernavskii, P.A.; Pankina, G.V. One-step synthesis, characterization and properties of novel hybrid electromagnetic nanomaterials based on polydiphenylamine and Co-Fe particles in the absence and presence of single-walled carbon nanotubes. RSC Adv. 2021, 11, 24772–24786. [Google Scholar] [CrossRef]
- Malekkiani, M.; Heshmati Jannat Magham, A.; Ravari, F.; Dadmehr, M. Facile fabrication of ternary MWCNTs/ZnO/chitosan nanocomposite for enhanced photocatalytic degradation of methylene blue and antibacterial activity. Sci. Rep. 2022, 12, 5927. [Google Scholar] [CrossRef]
- Baruah, B.; Kumar, A. PEDOT:PSS/MnO2/RGO ternary nanocomposite based anode catalyst for enhanced electrocatalytic activity of methanol oxidation for direct methanol fuel cell. Synth. Met. 2018, 245, 74–86. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Bordbar-Khiabani, A.; Ahangari-Asl, A. Fabrication of a ternary PANI@Fe3O4@CFs nanocomposite as a high performance electrode for solid-state supercapacitors. Int. J. Hydrogen Energy 2019, 44, 26794–26806. [Google Scholar] [CrossRef]
- Iqbal, J.; Ansari, M.O.; Numan, A.; Wageh, S.; Al-Ghamdi, A.; Alam, M.G.; Kumar, P.; Jafer, R.; Bashir, S.; Rajpar, A.H. Hydrothermally assisted synthesis of porous polyaniline@carbon nanotubes–manganese dioxide ternary composite for potential application in supercapattery. Polymers 2020, 12, 2918. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.T.; Ngan Tran, T.Q.; Ngo, D.-H.; Tai, D.C.; Kumar, S. Click-chemistry-mediated synthesis of silver nanoparticle-supported polymer-wrapped carbon nanotubes: Glucose sensor and antibacterial material. ACS Omega 2022, 7, 37095–37102. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Dhodamani, A.G.; Patil, S.M.; Mullani, S.B.; More, K.V.; Delekar, S.D. Interfacially interactive ternary silver-supported polyaniline/multiwalled carbon nanotube nanocomposites for catalytic and antibacterial activity. ACS Omega 2020, 5, 219–227. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Moses, J.C.; Gangrade, A.; Mandal, B.B. Carbon nanotubes and their polymer nanocomposites. In Nanomaterials and Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–175. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 6479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellino, M.; Rovere, M.; Shahzad, M.I.; Tagliaferro, A. Conductivity in carbon nanotube polymer composites: A comparison between model and experiment. Compos. Part A Appl. Sci. Manuf. 2016, 87, 237–242. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Advancement of a model for electrical conductivity of polymer nanocomposites reinforced with carbon nanotubes by a known model for thermal conductivity. Eng. Comput. 2022, 38, 2497–2507. [Google Scholar] [CrossRef]
- Alsultan, M.; Choi, J.; Wagner, P.; Swiegers, G.F. Synergistic amplification of oxygen generation in (photo)catalytic water splitting by a PEDOT/nano-Co3O4/MWCNT thin film composite. ChemCatChem 2020, 12, 1580–1584. [Google Scholar] [CrossRef]
- Ghasemi, A.K.; Ghorbani, M.; Lashkenari, M.S.; Nasiri, N. Facile synthesize of PANI/GO/CuFe2O4 nanocomposite material with synergistic effect for superb performance supercapacitor. Electrochim. Acta 2023, 439, 141685. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abdel-Aziz, M.H.; Zoromba, M.S.; Al-Hossainy, A.F. Structural, optical, and electrical properties of multi-walled carbon nanotubes/polyaniline/Fe3O4 ternary nanocomposites thin film. Synth. Met. 2018, 238, 1–13. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Yu, K.; Zhang, C.; Li, H.; Du, Z. Preparation of multi-walled carbon nanotube/polyaniline/Fe3O4 composites. Integr. Ferroelectr. 2014, 154, 159–165. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Dzidziguri, E.L.; Chernavskii, P.A.; Bondarenko, G.N.; Efimov, M.N.; Pankina, G.V. One-step synthesis of hybrid magnetic material based on polyphenoxazine and bimetallic Co-Fe nanoparticles. Polym. Bull. 2017, 74, 3043–3060. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Bondarenko, G.N. Phenoxazine polymers: Synthesis and structure. Rus. Chem. Bull. 2011, 60, 1651–1656. [Google Scholar] [CrossRef]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Baranchikov, A.E.; Karpacheva, G.P. IR radiation assisted preparation of KOH-activated polymer-derived carbon for methylene blue adsorption. J. Environ. Chem. Eng. 2019, 7, 103514. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Malakhov, A.O.; Karpacheva, G.P.; Bondarenko, G.; Marbelia, L.; Vankelecom, I.F.J.; Volkov, A.V. Creation of highlystable porous polyacrylonitrile membranes using infrared heating. React. Funct. Polym. 2021, 158, 104793. [Google Scholar] [CrossRef]
- Yan, X.; Suzuki, T.; Kitahama, Y.; Sato, H.; Itoh, T.; Ozaki, Y. A study on the interaction of single-walled carbon nanotubes (SWCNTs) and polystyrene (PS) at the interface in SWCNT–PS nanocomposites using tip-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 20618–20624. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Songca, S.P.; Rouxel, D.; Miska, P.; Lewu, F.B.; Kalarikkal, N.; Thomas, S. Completely green synthesis of silver nanoparticle decorated MWCNT and its antibacterial and catalytic properties. Pure Appl. Chem. 2016, 88, 71–81. [Google Scholar] [CrossRef]












| Materials | Co, % | Fe, % | C, % | N, % | H, % | O, % | C/N | C/H |
|---|---|---|---|---|---|---|---|---|
| PPOA | - | - | 78.7 | 7.7 | 4.9 | 8.7 | 10.2 | 16.1 |
| Co-Fe/PPOA | 11.4 | 10.3 | 53.5 | 5.5 | 1.8 | 17.5 | 9.7 | 29.7 |
| Co-Fe/SWCNT/PPOA | 10.2 | 7.8 | 56.9 | 4.8 | 1.6 | 18.7 | 11.9 | 35.6 |
| Co-Fe/MWCNT/PPOA | 14.5 | 14.1 | 55.3 | 4.3 | 1.4 | 10.4 | 12.9 | 39.5 |
| Nanomaterials | CCo *, wt % | CFe *, wt % | CCo **, % | CFe **, % | Co and Fe Phase Composition | HC, Oe | MS, emu/g | MR, emu/g | MR/MS |
|---|---|---|---|---|---|---|---|---|---|
| Co-Fe/PPOA | 5 | 10 | 7.2 | 12.8 | Co-Fe | 50 | 8.96 | 0.19 | 0.021 |
| Co-Fe/SWCNT/PPOA | 5 | 10 | 5.5 | 9.8 | Co-Fe | 75 | 22.14 | 0.75 | 0.034 |
| 5 | 20 | 4.9 | 16.3 | Co-Fe, Fe3O4, Fe4N | 50 | 41.82 | 0.91 | 0.022 | |
| 10 | 5 | 12.0 | 5.2 | Co-Fe, β-Co | 50 | 25.48 | 0.25 | 0.009 | |
| 10 | 10 | 10.2 | 7.8 | Co-Fe, Fe3O4 (traces) | 64 | 31.90 | 0.82 | 0.026 | |
| Co-Fe/MWCNT/PPOA | 5 | 10 | 6.8 | 11.1 | Co-Fe | 32 | 20.93 | 0.20 | 0.010 |
| 5 | 20 | 4.8 | 23.5 | Co-Fe, Fe3O4 | 37 | 44.33 | 0.50 | 0.011 | |
| 10 | 5 | 10.9 | 4.2 | Co-Fe, β-Co | 25 | 31.19 | 0.45 | 0.014 | |
| 10 | 10 | 14.5 | 14.1 | Co-Fe, Fe3O4 | 49 | 44.08 | 1.00 | 0.023 |
| Materials | CCo *, % | CFe *, % | Co and Fe Phase Composition | ∧ T5%, °C | ∧∧ T50%, °C | ∧∧∧ Residue, % |
|---|---|---|---|---|---|---|
| PPOA | - | - | - | 380/325 | 580/>1000 | 0/51 |
| Co-Fe/PPOA | 11.4 | 10.3 | Co-Fe | 134/131 | 670/>1000 | 28/64 |
| Co-Fe/SWCNT/PPOA | 10.2 | 7.8 | Co-Fe, Fe3O4 (traces) | 105/109 | 643/>1000 | 25/63 |
| Co-Fe/MWCNT/PPOA | 14.5 | 14.1 | Co-Fe, Fe3O4 | 93/97 | 563/>1000 | 34/70 |
| Materials | CCNT | * σac, S/cm | |
|---|---|---|---|
| PPOA | 0 | 9.73 × 10−10 | 8.78 × 10−6 |
| Co-Fe/PPOA | 0 | 9.67 × 10−3 | 9.71 × 10−3 |
| Co-Fe/SWCNT/PPOA | 3 | 3.16 × 10−2 | 3.17 × 10−2 |
| 10 | 5.94 × 10−1 | 5.96 × 10−1 | |
| Co-Fe/MWCNT/PPOA | 3 | 1.41 × 10−1 | 1.42 × 10−1 |
| 10 | 7.28 × 10−1 | 8.71 × 10−1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, S.; Petrov, V.; Vasilev, A.; Chernavskii, P.; Efimov, M.; Muratov, D.; Pankina, G.; Karpacheva, G. Formation Features of Polymer–Metal–Carbon Ternary Electromagnetic Nanocomposites Based on Polyphenoxazine. Polymers 2023, 15, 2894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15132894
Ozkan S, Petrov V, Vasilev A, Chernavskii P, Efimov M, Muratov D, Pankina G, Karpacheva G. Formation Features of Polymer–Metal–Carbon Ternary Electromagnetic Nanocomposites Based on Polyphenoxazine. Polymers. 2023; 15(13):2894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15132894
Chicago/Turabian StyleOzkan, Sveta, Valeriy Petrov, Andrey Vasilev, Petr Chernavskii, Mikhail Efimov, Dmitriy Muratov, Galina Pankina, and Galina Karpacheva. 2023. "Formation Features of Polymer–Metal–Carbon Ternary Electromagnetic Nanocomposites Based on Polyphenoxazine" Polymers 15, no. 13: 2894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15132894