Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences
Abstract
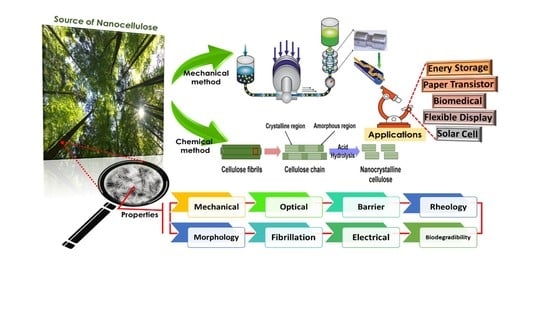
:1. Introduction
2. Classification of Nanocellulose
2.1. Cellulose Nanocrystals (CNCs) or Nanocrystalline Cellulose (NCC)
2.2. Cellulose Nanofibrils (CNFs) or Nanofibrillated Cellulose (NFC)
2.3. Bacterial Nanocellulose (BNC)
3. Production of Nanocellulose
3.1. Mechanical Method
3.1.1. Homogenization
3.1.2. Cryocrushing
3.1.3. Grinding
3.1.4. Microfluidization
3.1.5. Refining
3.1.6. Blending
3.1.7. Ball Milling
3.1.8. Aqueous Counter Collision (ACC)
3.2. Chemical Methods
3.2.1. Acid Hydrolysis
3.2.2. Alkaline Pretreatment
3.2.3. Oxidation Pretreatment
3.2.4. Enzymatic Pretreatment
3.2.5. Ionic Liquids
3.3. Physico-Mechanical Treatment
Ultrasonication
3.4. Chemico-Mechanical Treatment
Steam Explosion
3.5. Summary of Other Preparation Methods
4. Surface Modifications of Nanocellulose
| References | Effect of Surface Modification on Various Properties | Before Surface Modification | After Surface Modification | Reason |
|---|---|---|---|---|
| [141,142] | Crystallinity of nanocellulose | Lower crystalline value | Enhances the crystalline value | A greater hydrolysis time disintegration or remove the amorphous phase and improve the crystalline value |
| [143,144] | Toxicity of nanocellulose | Toxicity | As per the ecotoxicological evaluation, the nanocellulose has lower toxic and lower environmental damage | Proinflammatory and cytotoxicity reactions are minimizing toxicity |
| [145] | Specific surface area | Lower specific surface area (200–950 m2/g) | Excellent specific surface area (250−350 m2/g) | H2SO4 treatment |
| [146] | Aspect ratio | Low or medium aspect ratio | Higher aspect ratio | TEMPO oxidation method |
| [147,148] | Mechanical property | Poor mechanical property | Enhanced rigidity, strength, toughness, barrier features, and even flame retardancy | Collagen-based composite films reinforced with CNCs |
| [149] | Thermal property | Lower thermal expansion coefficient due to its higher crystallinity and strength of nanocellulose network | Excellent thermal property | H2SO4-hydrolyzed method |
| [142] | Rheological property | Tendency to shear-thinning and pseudoplasticity depends on the pH of the environment | Enhancement in shear rate with lower viscosity of nanocellulose | TEMPO oxidation method |
| [150] | Stability dispersion and agglomeration | Agglomeration and clustering of nanocellulose problem | Minimizes the agglomeration problem | Freeze-drying or supercritical drying of CO2 |
4.1. Noncovalent Surface Modification
4.2. Carbonylation
4.3. TEMPO-Mediated Oxidation
4.4. Esterification
4.5. Acetylation
4.6. Sulfonation
4.7. Summary of Nanocellulose Surface Modification
5. Structure–Property Correlation of Nanocellulose
5.1. Mechanical Properties
| References | Raw Material | Preparation Method | Max. Stress (MPa) | Modulus of Elasticity (GPa) |
|---|---|---|---|---|
| [184] | Softwood dissolving pulp | Vacuum filtering | 104 | 14.0 |
| [185] | Softwood and hardwood bleached kraft pulp | Vacuum filtering | 222–233 | 6.2–6.9 |
| [186] | Hardwood bleached kraft pulp | Vacuum filtering | 222–312 | 6.2–6.5 |
| [187] | Bleached spruce sulfite pulp | Vacuum filtering | 104–154 | 15.7–17.5 |
| [181] | Sugar beet pulp chips | Casting | 104 | 9.3 |
| [188,189] | Ramie | Retting | 393–870 | 7.3 |
| [188,190] | Cotton | Acidic hydrolysis | 128–597 | 5.5–12.6 |
| [191] | Kenaf | Retting | 930 | 53 |
| [192] | Jute | Retting | 393–800 | 10–30 |
| [193] | Banana | Chemical treatment | 600 | 17.85 |
| [194] | Bleached birch pulp | Mechanical disintegration | 172 | 5.3 |
| [195] | Bacterial nanocellulose | Not reported | 357.3 | 20.8 |
| [196] | A. xylinum | Two-step purification | 88.9 | 7.6 |
| [197] | Gelatin (A. xylinum) | Static cultivation | 63 | Not reported |
| [198] | Mulberry pulp | Acid hydrolysis | 33.3–41.3 | 0.77–1.11 |
| [199] | Tossa jute fiber | Acid hydrolysis | 32.94–48.66 | 4.81–5.76 |
| [200] | Softwood pulp | Ultrasonication | 141.6 | 12.27 |
| [200] | Algae | Ultrasonication | 77.97 | 8.12 |
| [201] | Cotton | Disc refiner | 23–26 | Not reported |
5.2. Optical Properties
5.3. Barrier Properties
5.4. Rheology of Nanocellulose
| References | Raw Material | Shear Rate (s−1) | Viscosity | Run Temp. (°C) |
|---|---|---|---|---|
| [211] | Pineapple | 22.2 | 3.5 × 104 Pa.s | 125 |
| [212] | Softwood sulfite pulp | 20 | 260 mPa.s | 20 |
| [213] | Cellulose nanofibrils | 0.1–1.0 | 10–100 mPa.s | 25 |
| [214] | Kenaf/PLA | 103–104 | 50–300 Pa.s | 200 |
| [215] | Jute/PP | 10−2–104 | 10–104 Pa.s | 180 |
| [216] | Hemp/PP | 10−1–103 | 102–105 Pa.s | 180 |
| [217] | Gluconacetobacter xylinus | 0–400 | 170–400 Pa.s | 25 |
5.5. Morphology
5.6. Degree of Fibrillation
5.7. Electrical Properties
| References | Nanocellulose Type | Conductive Structure | Conductivity (S cm−1) |
|---|---|---|---|
| [231] | CNCs | PPy | Up to 36 |
| [232] | CNFs | PPy | 1.5 |
| [233] | CNCs | PANI | Up to 10−1 |
| [234] | CNFs | PANI | 2.6 × 10−5 |
| [235] | CNFs | Silver | 5 |
| [236] | CNCs | PANI + PFE | 0.01–0.5 |
| [237] | CNCs | PPy | Up to 4 |
| [238] | CNCs | PANI | 2.6 × 10−5 |
| [239] | BC | CNT | 0.13 × 10−3 |
| [194] | CNFs | GO | 7.3 × 10−2 –15.4 |
| [195] | BC | PANI | 2.0 × 10−4–9.5 × 10−3 |
5.8. Biodegradability
6. Applications of Nanocellulose
6.1. Biomedical
6.2. Flexible Display
6.3. Energy Storage
6.4. Paper Transistor
6.5. Solar Cells
6.6. Overview of Nanocellulose Applications
7. Future Perspectives and Challenges
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |||
| CNCs | Cellulose nanocrystals | MCC | Microcrystalline cellulose |
| CNFs | Cellulose nanofibrils | ACC | Aqueous counter collision |
| BNC | Bacterial nanocellulose | ILs | Ionic liquids |
| SEM | Scanning electron microscope | HIUS | High-intensity ultrasonication |
| TEM | Transmission electron microscope | WAXS | Wide-angle X-ray scattering |
| CAGR | Compound annual growth rate | PLA | Polylactic acid |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1yl)oxyl | AFM | Atomic force microscopy |
| NaOCl | Sodium hypochlorite | IPTS | Isocyanatepropltriethoxysilane |
| CAGA | Compound annual growth rate | USA | United States of America |
| PVA | Polyvinyl alcohol | OLED | Organic light-emitting diode |
| LIB | Li-ion battery | Symbols | |
| MWCNT | Multiwalled carbon nanotube | USD | United States dollar |
| IgG | Immunoglobulin | μ | Micro |
| PANI | Polyaniline | Å | Angstrom |
| CTE | Coefficient of thermal expansion | nm | Nanometer |
| APS | Ammonium persulfate | ppm | Parts per million |
References
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of nanocellulose and its application in nanocomposite packaging material: A review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W.; Zhang, X.; Lei, T.; Lee, S.-Y.; Wu, Q. ZIF-67@ Cellulose nanofiber hybrid membrane with controlled porosity for use as Li-ion battery separator. J. Energy Chem. 2021, 52, 170–180. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361. [Google Scholar] [CrossRef]
- Hentze, H.-P. From nanocellulose science towards applications. Dev. Adv. Biocompos. 2010, 71, 71–96. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 76. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Balat, M.; Ayar, G. Biomass energy in the world, use of biomass and potential trends. Energy Sources 2005, 27, 931–940. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.; Tam, K. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-Based Nanocomposites for Sustainable Applications: A Review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, P.; Singh, P.P.; Vo, D.-V.N.; Kumar, D.; Balasubramanian, K.; Mubayi, A.; Srivastava, A.; Sharma, A. Bacterial nanocellulose: Green polymer materials for high performance energy storage applications. J. Environ. Chem. Eng. 2022, 10, 108176. [Google Scholar] [CrossRef]
- Shen, Z.; Qin, M.; Xiong, F.; Zou, R.; Zhang, J. Nanocellulose-based composite phase change materials for thermal energy storage: Status and challenges. Energy Environ. Sci. 2023, 16, 830–861. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly (ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkazemi, F.; Doosthoseini, K.; Ganjian, E.; Azin, M. Manufacturing of bacterial nano-cellulose reinforced fiber− cement composites. Constr. Build. Mater. 2015, 101, 958–964. [Google Scholar] [CrossRef]
- Wang, H.D.; Roeder, R.D.; Whitney, R.A.; Champagne, P.; Cunningham, M.F. Graft modification of crystalline nanocellulose by Cu (0)-mediated SET living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2800–2808. [Google Scholar] [CrossRef]
- Milanez, D.H.; Amaral, R.M.d.; Faria, L.I.L.d.; Gregolin, J.A.R. Assessing nanocellulose developments using science and technology indicators. Mater. Res. 2013, 16, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J. Multifunctional bionanocomposite films of poly (lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly [2-(dimethylamino) ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef]
- Hu, Z.; Marway, H.S.; Kasem, H.; Pelton, R.; Cranston, E.D. Dried and redispersible cellulose nanocrystal Pickering emulsions. ACS Macro Lett. 2016, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Finazzi, M.; Tavazzi, S.; Piergiovanni, L. Tunable green oxygen barrier through layer-by-layer self-assembly of chitosan and cellulose nanocrystals. Carbohydr. Polym. 2013, 92, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Sui, L.; Elkasabi, Y.; Burgardt, P.; Lee, J.; Miryala, A.; Kusumaatmaja, W.; Carman, M.R.; Shtein, M.; Kieffer, J. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir 2007, 23, 7901–7906. [Google Scholar] [CrossRef] [PubMed]
- Cerclier, C.; Guyomard-Lack, A.; Moreau, C.; Cousin, F.; Beury, N.; Bonnin, E.; Jean, B.; Cathala, B. Coloured Semi-reflective Thin Films for Biomass-hydrolyzing Enzyme Detection. Adv. Mater. 2011, 23, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lai, C.; Marchewka, R.; Berry, R.; Tam, K. Use of CdS quantum dot-functionalized cellulose nanocrystal films for anti-counterfeiting applications. Nanoscale 2016, 8, 13288–13296. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J. Mater. Chem. 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- Roco, M.C.; Bainbridge, W.S. Societal implications of nanoscience and nanotechnology: Maximizing human benefit. J. Nanopart. Res. 2005, 7, 1–13. [Google Scholar] [CrossRef]
- Buch, N.; Rehman, O.; Hiller, J. Impact of processed cellulose fibers on portland cement concrete properties. Transp. Res. Rec. J. Transp. Res. Board 1999, 1668, 72–80. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial cellulose II. Processing of the gelatinous cellulose for food materials. Food Hydrocoll. 1992, 6, 479–487. [Google Scholar] [CrossRef]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Watanabe, K.; Eto, Y.; Takano, S.; Nakamori, S.; Shibai, H.; Yamanaka, S. A new bacterial cellulose substrate for mammalian cell culture. Cytotechnology 1993, 13, 107–114. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2006, 76, 431–438. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Chandana, A.; Mallick, S.P.; Dikshit, P.K.; Singh, B.N.; Sahi, A.K. Recent developments in bacterial nanocellulose production and its biomedical applications. J. Polym. Environ. 2022, 30, 4040–4067. [Google Scholar] [CrossRef]
- Yasuda, K.; Gong, J.P.; Katsuyama, Y.; Nakayama, A.; Tanabe, Y.; Kondo, E.; Ueno, M.; Osada, Y. Biomechanical properties of high-toughness double network hydrogels. Biomaterials 2005, 26, 4468–4475. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Jia, S.; Huang, Y.; Gao, C.; Wan, Y. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.; Othman, N.E.A.; Wahab, N.A.; Aziz, A.A. The Effect of Chemical and High Pressure Homogenization Treatment Conditions on the Morphology of Nanocellulose. J. Adv. Res. Appl. Mech. 2022, 93, 1–7. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 7923068–7923092. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sain, M.; Kortschot, M. Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing. Holzforschung 2005, 59, 102–107. [Google Scholar] [CrossRef]
- Sihag, S.; Pal, J.; Yadav, M. Extraction and Characterization of Nanocellulose from Wheat Straw: Facile Approach. J. Water Environ. Nanotechnol. 2022, 7, 317–331. [Google Scholar]
- Bhatnagar, A.; Sain, M. Processing of cellulose nanofiber-reinforced composites. J. Reinf. Plast. Compos. 2005, 24, 1259–1268. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Dispersion of soybean stock-based nanofiber in a plastic matrix. Polym. Int. 2007, 56, 538–546. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 2007, 67, 2521–2527. [Google Scholar] [CrossRef]
- Gulipalli, P.; Borle, S.; Chivukula, K.; Adusumalli, R.B. Processing of nanocellulose sheet for capturing fine particulate matter. Mater. Today Proc. 2022, 72, 402–409. [Google Scholar] [CrossRef]
- Taniguchi, T.; Okamura, K. New films produced from microfibrillated natural fibres. Polym. Int. 1998, 47, 291–294. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A Mater. Sci. Process. 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.; Gleisner, R.; Kuster, T.; Baxa, U.; McNeil, S. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M. Preparation of black cumin seed oil Pickering nanoemulsion with enhanced stability and antioxidant potential using nanocrystalline cellulose from oil palm empty fruit bunch. Chemosphere 2022, 287, 132108. [Google Scholar] [CrossRef]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Ind. Crop. Prod. 2016, 84, 337–343. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.; Patil, S.; Nadanathangam, V.; Saxena, S. Development of energy efficient nanocellulose production process by enzymatic pretreatment and controlled temperature refining of cotton linters. Cellulose 2022, 30, 833–847. [Google Scholar] [CrossRef]
- Hamada, H.; Tahara, K.; Uchida, A. The effects of nano-fibrillated cellulose as a coating agent for screen printing. In Proceedings of the 12th TAPPI Advanced Coating Fundamentals Symposium, Atlanta, GA, USA, 10–12 September 2012. [Google Scholar]
- Nakagaito, A.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A. Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J. Appl. Polym. Sci. 2007, 106, 2817–2824. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated Cellulose. U.S. Patent No. 4,374,702, 22 February 1983. [Google Scholar]
- Joseleau, J.-P.; Chevalier-Billosta, V.; Ruel, K. Interaction between microfibrillar cellulose fines and fibers: Influence on pulp qualities and paper sheet properties. Cellulose 2012, 19, 769–777. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Hassan, M.L.; Hassan, E.A.; Oksman, K.N. Effect of pretreatment of bagasse fibers on the properties of chitosan/microfibrillated cellulose nanocomposites. J. Mater. Sci. 2011, 46, 1732–1740. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Karande, V.; Bharimalla, A.; Hadge, G.; Mhaske, S.; Vigneshwaran, N. Nanofibrillation of cotton fibers by disc refiner and its characterization. Fibers Polym. 2011, 12, 399–404. [Google Scholar] [CrossRef]
- Bharimalla, A.; Patil, P.; Deshmukh, S.; Vigneshwaran, N. Energy efficient production of nano-fibrillated cellulose (nfc) from cotton linters by tri-disc refining and its characterization. Cellul. Chem. Technol. 2017, 51, 395–401. [Google Scholar]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Ikenaga, K.; Takagi, H. Cellulose nanofiber extraction from grass by a modified kitchen blender. Mod. Phys. Lett. B 2015, 29, 1540039. [Google Scholar] [CrossRef]
- Verma, Y.K.; Singh, A.K.; Paswan, M. Preparation of Bamboo-Based Nano-Cellulose by Ball Milling. In Advances in Material Science and Metallurgy; Springer: Berlin/Heidelberg, Germany, 2023; pp. 89–98. [Google Scholar]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Kekäläinen, K.; Liimatainen, H.; Biale, F.; Niinimäki, J. Nanofibrillation of TEMPO-oxidized bleached hardwood kraft cellulose at high solids content. Holzforschung 2015, 69, 1077–1088. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Wang, Y.; Yan, J. Energy transfer and kinetics in mechanochemistry. Environ. Sci. Pollut. Res. 2017, 24, 24562–24571. [Google Scholar] [CrossRef]
- Tatsumi, D.; Kanda, A.; Kondo, T. Characterization of mercerized cellulose nanofibrils prepared by aqueous counter collision process. J. Wood Sci. 2022, 68, 13. [Google Scholar] [CrossRef]
- Kose, R.; Mitani, I.; Kasai, W.; Kondo, T. “Nanocellulose” as a single nanofiber prepared from pellicle secreted by gluconacetobacter xylinus using aqueous counter collision. Biomacromolecules 2011, 12, 716–720. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polym. 2014, 112, 284–290. [Google Scholar] [CrossRef]
- Tsuboi, K.; Yokota, S.; Kondo, T. Difference between bamboo-and wood-derived cellulose nanofibers prepared by the aqueous counter collision method. Nord. Pulp Pap. Res. J. 2014, 29, 69–76. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. The effect of chemically coated nanofiber reinforcement on biopolymer based nanocomposites. Bioresources 2007, 2, 371–388. [Google Scholar]
- Wang, B.; Sain, M.; Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl. Compos. Mater. 2007, 14, 89. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hamid, S.B.A.; Zain, S. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Vignon, M.R. Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 2008, 15, 177–185. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Targeted disruption of hydroxyl chemistry and crystallinity in natural fibers for the isolation of cellulose nano-fibers via enzymatic treatment. BioResources 2011, 6, 1242–1250. [Google Scholar]
- López-Rubio, A.; Lagaron, J.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr. Polym. 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Quero, F.; Nogi, M.; Yano, H.; Young, R.J.; Lindström, T.; Sampson, W.W.; Eichhorn, S.J. Effective Young’s modulus of bacterial and microfibrillated cellulose fibrils in fibrous networks. Biomacromolecules 2012, 13, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Kuzmina Olga, G.; Sashina Elena, S.; Svetlana, T.; Dariusz, W. Dissolved state of cellulose in ionic liquids-the impact of water. Fibres Text. East. Eur. 2010, 18, 32–37. [Google Scholar]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Mohd Ishak, N.A.; Abdullah, F.Z.; Muhd Julkapli, N. Production and characteristics of nanocellulose obtained with using of ionic liquid and ultrasonication. J. Nanopart. Res. 2022, 24, 171. [Google Scholar] [CrossRef]
- Abramov, O. Theory and Industrial Applications, Gordon and Breach Science Publishers. In High Intensity Ultrasonics; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Wang, S.; Cheng, Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J. Appl. Polym. Sci. 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Gemmer, R.E.; Borsoi, C.; Hansen, B.; Dahlem Júnior, M.A.; Francisquetti, E.L.; Rossa Beltrami, L.V.; Zattera, A.J.; Catto, A.L. Extraction of Nanocellulose from Yerba Mate Residues Using Steam Explosion, TEMPO-mediated Oxidation and Ultra-fine Friction Grinding. J. Nat. Fibers 2022, 19, 10539–10549. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Yen, F.-Y.; Hung, F.; Su, C.-H.; Chen, W.-H. Intermittent pressurized operation of steam explosion pretreatment system. J. Taiwan Inst. Chem. Eng. 2016, 67, 285–291. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Savadekar, N.; Mhaske, S. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Zain, S.K.; Das, R.; Centi, G. Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr. Polym. 2016, 138, 349–355. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Christma, F.A.; Toyin, A.J.; Gopinath, S.C.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Benini, K.C.C.d.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef]
- Wang, H.; Kong, L.; Ziegler, G.R. Fabrication of starch—Nanocellulose composite fibers by electrospinning. Food Hydrocoll. 2019, 90, 90–98. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Zdunek, A. Tailored nanocellulose structure depending on the origin. Example of apple parenchyma and carrot root celluloses. Carbohydr. Polym. 2019, 210, 186–195. [Google Scholar] [CrossRef]
- Franco, T.S.; Potulski, D.C.; Viana, L.C.; Forville, E.; de Andrade, A.S.; de Muniz, G.I.B. Nanocellulose obtained from residues of peach palm extraction (Bactris gasipaes). Carbohydr. Polym. 2019, 218, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hse, C.-Y.; Cornelis, F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Chandra, C.S.J.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar]
- Oliveira, F.B.d.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.d.S.; Belgacem, M.N. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind. Crop. Prod. 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT-Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Huang, J.; Ma, X.; Yang, G.; Alain, D. Introduction to nanocellulose. Nanocellul. Fundam. Adv. Mater. 2019, 8, 392. [Google Scholar]
- Lu, J.; Sun, C.; Yang, K.; Wang, K.; Jiang, Y.; Tusiime, R.; Yang, Y.; Fan, F.; Sun, Z.; Liu, Y. Properties of polylactic acid reinforced by hydroxyapatite modified nanocellulose. Polymers 2019, 11, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Wu, C.; Zhang, Y.; Cao, X.; Luo, Z. Microstructure and thermal and tensile properties of poly (vinyl alcohol) nanocomposite films reinforced by polyacrylamide grafted cellulose nanocrystals. J. Macromol. Sci. Part B 2020, 59, 223–234. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Luo, J.; Low, M.Y.; Shi, Z.; Tang, J.; Zhang, Z.; Peng, B.; Tam, K.C. Pickering emulsions stabilized by hydrophobically modified nanocellulose containing various structural characteristics. Cellulose 2019, 26, 7753–7767. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose processing properties and potential applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the potential biological impact of cellulose nanocrystals. Fibers 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.; Viet, D.; Bruzzese, C.; Dufresne, A. Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions. Carbohydr. Polym. 2011, 84, 211–215. [Google Scholar] [CrossRef]
- Sattar, M.A.; Patnaik, A. Role of interface structure and chain dynamics on the diverging glass transition behavior of SSBR-SiO2-PIL elastomers. ACS Omega 2020, 5, 21191–21202. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Zhou, Q.; Brumer, H.; Teeri, T.T. Self-Organization of Cellulose Nanocrystals Adsorbed with Xyloglucan Oligosaccharide—Poly (ethylene glycol)—Polystyrene Triblock Copolymer. Macromolecules 2009, 42, 5430–5432. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Marais, S.; Dufresne, A. Water transport properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. J. Membr. Sci. 2013, 427, 218–229. [Google Scholar] [CrossRef]
- De Nooy, A.; Besemer, A.; Van Bekkum, H. Highly selective TEMPO mediated oxidation of primary alcohol groups in polysaccharides. Recl. Trav. Chim. Pays-Bas 1994, 113, 165–166. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2011, 18, 257–270. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.; Agarwal, U.; Cai, Z.; Wu, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr. Polym. 2013, 97, 226–234. [Google Scholar] [CrossRef]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Fathi, M.; Karim, M.; Ahmadi, N. Nanostructures of cellulose for encapsulation of food ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 493–519. [Google Scholar]
- Chin, K.M.; Sung Ting, S.; Ong, H.L.; Omar, M. Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review. J. Appl. Polym. Sci. 2018, 135, 46065. [Google Scholar] [CrossRef] [Green Version]
- Eyholzer, C.; Tingaut, P.; Zimmermann, T.; Oksman, K. Dispersion and reinforcing potential of carboxymethylated nanofibrillated cellulose powders modified with 1-hexanol in extruded poly (lactic acid) (PLA) composites. J. Polym. Environ. 2012, 20, 1052–1062. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Eyholzer, C.; Lopez-Suevos, F.; Tingaut, P.; Zimmermann, T.; Oksman, K. Reinforcing effect of carboxymethylated nanofibrillated cellulose powder on hydroxypropyl cellulose. Cellulose 2010, 17, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Daud, J.B.; Lee, K.Y. Surface modification of nanocellulose. Handb. Nanocellul. Cellul. Nanocompos. 2017, 1, 101–122. [Google Scholar]
- Nechita, P.; Panaitescu, D. Improving the dispersibility of cellulose microfibrillated structures in polymer matrix by controlling drying conditions and chemical surface modifications. Cell Chem. Technol. 2013, 47, 711–719. [Google Scholar]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr. Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, N. Surface chemistry of nanocellulose. Nanocellul. Fundam. Adv. Mater. 2019, 5, 115–153. [Google Scholar]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirviö, J.A.; Hormi, O.E.; Niinimaki, J. Enhancement of the nanofibrillation of wood cellulose through sequential periodate–chlorite oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Semenikhin, N.; Chang, H.; Moon, R.J.; Kumar, S. Post-sulfonation of cellulose nanofibrils with a one-step reaction to improve dispersibility. Carbohydr. Polym. 2018, 181, 247–255. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Ferreira, F.; Mariano, M.; Rabelo, S.; Gouveia, R.; Lona, L. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro-to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Syverud, K.; Chinga-Carrasco, G.; Toledo, J.; Toledo, P.G. A comparative study of Eucalyptus and Pinus radiata pulp fibres as raw materials for production of cellulose nanofibrils. Carbohydr. Polym. 2011, 84, 1033–1038. [Google Scholar] [CrossRef]
- Sassi, J.-F.; Chanzy, H. Ultrastructural aspects of the acetylation of cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Z.; Wang, C.; Wang, A.; Liu, X.; Wei, B.; Wen, Y. Enhancing the redispersibility of TEMPO-mediated oxidized cellulose nanofibrils in N, N-dimethylformamide by modification with cetyltrimethylammonium bromide. Cellulose 2019, 26, 7769–7780. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Leitner, J.; Hinterstoisser, B.; Wastyn, M.; Keckes, J.; Gindl, W. Sugar beet cellulose nanofibril-reinforced composites. Cellulose 2007, 14, 419–425. [Google Scholar] [CrossRef]
- Bruce, D.; Hobson, R.; Farrent, J.; Hepworth, D. High-performance composites from low-cost plant primary cell walls. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1486–1493. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaille, J.-Y.; Vignon, M.R. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J. Appl. Polym. Sci. 1997, 64, 1185–1194. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2009, 16, 75. [Google Scholar] [CrossRef]
- Junior, C.P.; De Carvalho, L.; Fonseca, V.; Monteiro, S.; d’Almeida, J. Analysis of the tensile strength of polyester/hybrid ramie–cotton fabric composites. Polym. Test. 2004, 23, 131–135. [Google Scholar] [CrossRef]
- Stevens, C. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 10. [Google Scholar]
- Morais, J.P.S.; de Freitas Rosa, M.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Nishino, T.; Hirao, K.; Kotera, M.; Nakamae, K.; Inagaki, H. Kenaf reinforced biodegradable composite. Compos. Sci. Technol. 2003, 63, 1281–1286. [Google Scholar] [CrossRef]
- Rao, D.V.; Srinivas, K.; Naidu, A.L. A review on Jute stem Fiber and its Composites. Int. J. Eng. Trends Technol. 2017, 6, 1–9. [Google Scholar]
- Rao, P.D.; Rao, D.V.; Naidu, A.L.; Bahubalendruni, M.R. Mechanical Properties of Banana fiber Reinforced Composites and Manufacturing Techniques: A Review. Int. J. Res. Dev. Technol. 2017, 8, 39–46. [Google Scholar]
- Dang, L.N.; Seppälä, J. Electrically conductive nanocellulose/graphene composites exhibiting improved mechanical properties in high-moisture condition. Cellulose 2015, 22, 1799–1812. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Seetabhawang, S.; Siripong, P.; Phisalaphong, M. Biosynthesis and characterization of nanocellulose-gelatin films. Materials 2013, 6, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Militký, J.; Wiener, J.; Kale, B.M.; Ali, U.; Rwawiire, S. Nanocellulose coated woven jute/green epoxy composites: Characterization of mechanical and dynamic mechanical behavior. Compos. Struct. 2017, 161, 340–349. [Google Scholar] [CrossRef]
- Le Bras, D.; Strømme, M.; Mihranyan, A. Characterization of Dielectric Properties of Nanocellulose from Wood and Algae for Electrical Insulator Applications. J. Phys. Chem. B 2015, 119, 5911–5917. [Google Scholar] [CrossRef]
- Savadekar, N.; Karande, V.; Vigneshwaran, N.; Bharimalla, A.; Mhaske, S. Preparation of nano cellulose fibers and its application in kappa-carrageenan based film. Int. J. Biol. Macromol. 2012, 51, 1008–1013. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Characterization of microfibrillated cellulose (MFC) films made of different types of raw material. Nord. Polym. Days 2008, 5, 11–13. [Google Scholar]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Nogi, M.; Yano, H. Optically transparent nanofiber sheets by deposition of transparent materials: A concept for a roll-to-roll processing. Appl. Phys. Lett. 2009, 94, 233117. [Google Scholar]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Osterberg, M.; Wågberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures.Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Dufresne, A.; Dupeyre, D.; Vignon, M.R. Cellulose microfibrils from potato tuber cells: Processing and characterization of starch–cellulose microfibril composites. J. Appl. Polym. Sci. 2000, 76, 2080–2092. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Roux, D.; Nishiyama, Y. Rheological properties of microfibrillar suspension of TEMPO-oxidized pulp. Cellulose 2008, 15, 425–433. [Google Scholar] [CrossRef]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using Raman spectroscopy. Appl. Phys. Lett. 2008, 93, 033111. [Google Scholar] [CrossRef]
- George, J.; Janardhan, R.; Anand, J.; Bhagawan, S.; Thomas, S. Melt rheological behaviour of short pineapple fibre reinforced low density polyethylene composites. Polymer 1996, 37, 5421–5431. [Google Scholar] [CrossRef]
- Colson, J.; Bauer, W.; Mayr, M.; Fischer, W.; Gindl-Altmutter, W. Morphology and rheology of cellulose nanofibrils derived from mixtures of pulp fibres and papermaking fines. Cellulose 2016, 23, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Serizawa, S.; Inoue, K.; Iji, M. Kenaf-fiber-reinforced poly (lactic acid) used for electronic products. J. Appl. Polym. Sci. 2006, 100, 618–624. [Google Scholar] [CrossRef]
- Mohanty, S.; Verma, S.K.; Nayak, S.K. Rheological characterization of PP/jute composite melts. J. Appl. Polym. Sci. 2006, 99, 1476–1484. [Google Scholar] [CrossRef]
- Twite-Kabamba, E.; Mechraoui, A.; Rodrigue, D. Rheological properties of polypropylene/hemp fiber composites. Polym. Compos. 2009, 30, 1401–1407. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, H.; Sun, H.-J.; Zhao, Y.; Yang, X.; Cheng, S.Z.; Yang, G. Thermoresponsive bacterial cellulose whisker/poly (NIPAM-co-BMA) nanogel complexes: Synthesis, characterization, and biological evaluation. Biomacromolecules 2013, 14, 1078–1084. [Google Scholar] [CrossRef]
- Liimatainen, H.; Ezekiel, N.; Sliz, R.; Ohenoja, K.; Sirviö, J.A.; Berglund, L.; Hormi, O.; Niinimäki, J. High-strength nanocellulose–talc hybrid barrier films. ACS Appl. Mater. Interfaces 2013, 5, 13412–13418. [Google Scholar] [CrossRef]
- Olszewska, A.; Eronen, P.; Johansson, L.-S.; Malho, J.-M.; Ankerfors, M.; Lindström, T.; Ruokolainen, J.; Laine, J.; Österberg, M. The behaviour of cationic nanofibrillar cellulose in aqueous media. Cellulose 2011, 18, 1213. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Pignon, F.; Belgacem, M.N. Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process. J. Mater. Sci. 2015, 50, 531–541. [Google Scholar] [CrossRef]
- Besbes, I.; Vilar, M.R.; Boufi, S. Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: Preparation, characteristics and reinforcing potential. Carbohydr. Polym. 2011, 86, 1198–1206. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Optical methods for the quantification of the fibrillation degree of bleached MFC materials. Micron 2013, 48, 42–48. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef]
- El Baradai, O.; Beneventi, D.; Alloin, F.; Bongiovanni, R.; Bruas-Reverdy, N.; Bultel, Y.; Chaussy, D. Microfibrillated cellulose based ink for eco-sustainable screen printed flexible electrodes in lithium ion batteries. J. Mater. Sci. Technol. 2016, 32, 566–572. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-paper flexible Li-ion battery cells through a paper-making process based on nano-fibrillated cellulose. J. Mater. Chem. A 2013, 1, 4671–4677. [Google Scholar] [CrossRef] [Green Version]
- Flandin, L.; Cavaillé, J.; Bidan, G.; Brechet, Y. New nanocomposite materials made of an insulating matrix and conducting fillers: Processing and properties. Polym. Compos. 2000, 21, 165–174. [Google Scholar] [CrossRef]
- Schroers, M.; Kokil, A.; Weder, C. Solid polymer electrolytes based on nanocomposites of ethylene oxide–epichlorohydrin copolymers and cellulose whiskers. J. Appl. Polym. Sci. 2004, 93, 2883–2888. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Wu, X.; Wang, S.; Lu, C. Cellulose nanocrystals mediated assembly of graphene in rubber composites for chemical sensing applications. Carbohydr. Polym. 2016, 140, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, X.; Wu, X.; Lu, C. Tailoring percolating conductive networks of natural rubber composites for flexible strain sensors via a cellulose nanocrystal templated assembly. Soft Matter 2016, 12, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, J.; Duan, Y.; Yu, A.; Berry, R.M.; Tam, K.C. Conductive cellulose nanocrystals with high cycling stability for supercapacitor applications. J. Mater. Chem. A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Rubino, S.; Razaq, A.; Nyholm, L.; Strømme, M.; Leifer, K.; Mihranyan, A. Spatial mapping of elemental distributions in polypyrrole-cellulose nanofibers using energy-filtered transmission electron microscopy. J. Phys. Chem. B 2010, 114, 13644–13649. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Sanches, A.O.; Malmonge, L.F.; Medeiros, E.S.; Rosa, M.F.; McMahan, C.M.; Malmonge, J.A. Conductive Nanocomposites Based on Cellulose Nanofibrils Coated with Polyaniline-DBSA Via In Situ Polymerization. In Macromolecular Symposia; Wiley-Vch Verlag: Weinheim, Germany, 2012; pp. 196–202. [Google Scholar]
- Luong, N.D.; Korhonen, J.T.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.-S.; Seppälä, J. Processable polyaniline suspensions through in situ polymerization onto nanocellulose. Eur. Polym. J. 2013, 49, 335–344. [Google Scholar] [CrossRef]
- Wu, M.; Kuga, S.; Huang, Y. Quasi-one-dimensional arrangement of silver nanoparticles templated by cellulose microfibrils. Langmuir 2008, 24, 10494–10497. [Google Scholar] [CrossRef]
- van den Berg, O.; Schroeter, M.; Capadona, J.R.; Weder, C. Nanocomposites based on cellulose whiskers and (semi) conducting conjugated polymers. J. Mater. Chem. 2007, 17, 2746–2753. [Google Scholar] [CrossRef]
- Wu, X.; Chabot, V.L.; Kim, B.K.; Yu, A.; Berry, R.M.; Tam, K.C. Cost-effective and scalable chemical synthesis of conductive cellulose nanocrystals for high-performance supercapacitors. Electrochim. Acta 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sui, G.; Bhattacharyya, D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos. Sci. Technol. 2014, 99, 31–36. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Cheng, J.; Park, M.; Hyun, J. Amphiphilic comb-like polymer for harvest of conductive nano-cellulose. Colloids Surf. B Biointerfaces 2012, 89, 161–166. [Google Scholar] [CrossRef]
- Abraham, E.; Elbi, P.; Deepa, B.; Jyotishkumar, P.; Pothen, L.; Narine, S.; Thomas, S. X-ray diffraction and biodegradation analysis of green composites of natural rubber/nanocellulose. Polym. Degrad. Stab. 2012, 97, 2378–2387. [Google Scholar] [CrossRef]
- Abdel-Hakim, A.; Mouradb, R.M. Nanocellulose and its polymer composites: Preparation, characterization, and applications. Russ. Chem. Rev. 2023, 92, 4. [Google Scholar]
- Zhang, Y.; Deng, W.; Wu, M.; Rahmaninia, M.; Xu, C.; Li, B. Tailoring Functionality of Nanocellulose: Current Status and Critical Challenges. Nanomaterials 2023, 13, 1489. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sood, A.; Han, S.S. Potential of magnetic nano cellulose in biomedical applications: Recent Advances. Biomater. Polym. Horiz. 2022, 1, 32–47. [Google Scholar] [CrossRef]
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.M.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 231, 123316. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ruottinen, V.; Ruotsalainen, J.; Rönkkö, S.; Johansson, L.-S.; Hinkkanen, A.; Järvinen, K.; Seppälä, J. Synthesis of cellulose nanocrystals carrying tyrosine sulfate mimetic ligands and inhibition of alphavirus infection. Biomacromolecules 2014, 15, 1534–1542. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; French, A.; Concha, M.; DeLucca, A.; Wu, Q. Nanocellulose-based biosensors: Design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having fluorimetric and colorimetric elastase detection sensitivity. Engineering 2013, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.V.; Prevost, N.; Sethumadhavan, K.; Ullah, A.; Condon, B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 2013, 20, 1223–1235. [Google Scholar] [CrossRef]
- Ullah, M.W.; Adhikari, M.; Atta, O.M.; Farooq, U.; Ul-Islam, M.; Shahzad, A.; Manan, S.; Yang, G. Biomedical Applications of Nanocellulose. In Emerging Nanotechnologies in Nanocellulose; Springer: Berlin/Heidelberg, Germany, 2023; pp. 367–406. [Google Scholar]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface functionalized nanofibrillar cellulose (NFC) film as a platform for immunoassays and diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Carbonell, R.G.; Rojas, O.J. Bioactive cellulose nanofibrils for specific human IgG binding. Biomacromolecules 2013, 14, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. J. Renew. Mater. 2013, 1, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, J.; Zhong, J. Nanocellulose Paper for Flexible Electronic Substrate. In Emerging Nanotechnologies in Nanocellulose; Springer: Berlin/Heidelberg, Germany, 2023; pp. 211–235. [Google Scholar]
- Xie, J.; Jia, D.; Dirican, M.; Xia, Y.; Li, C.; Liu, Y.; Cui, M.; Yan, C.; Wan, J.; Liu, H. Highly foldable, super-sensitive, and transparent nanocellulose/ceramic/polymer cover windows for flexible OLED displays. ACS Appl. Mater. Interfaces 2022, 14, 16658–16668. [Google Scholar] [CrossRef]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood–cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Faraco, T.A.; Fontes, M.d.L.; Paschoalin, R.T.; Claro, A.M.; Gonçalves, I.S.; Cavicchioli, M.; Farias, R.L.D.; Cremona, M.; Ribeiro, S.J.L.; Barud, H.d.S. Review of Bacterial Nanocellulose as Suitable Substrate for Conformable and Flexible Organic Light-Emitting Diodes. Polymers 2023, 15, 479. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Juntaro, J.; Sain, M.; Manuspiya, H. Development of transparent bacterial cellulose nanocomposite film as substrate for flexible organic light emitting diode (OLED) display. Ind. Crop. Prod. 2012, 35, 92–97. [Google Scholar] [CrossRef]
- Sabo, R.; Yermakov, A.; Law, C.T.; Elhajjar, R. Nanocellulose-enabled electronics, energy harvesting devices, smart materials and sensors: A review. J. Renew. Mater. 2016, 4, 297–312. [Google Scholar] [CrossRef]
- Wang, Z.; Nyholm, L. Energy Storage Applications. In Emerging Nanotechnologies in Nanocellulose; Springer: Berlin/Heidelberg, Germany, 2023; pp. 237–265. [Google Scholar]
- Sun, Z.; Eyley, S.; Guo, Y.; Salminen, R.; Thielemans, W. Synergistic effects of chloride anions and carboxylated cellulose nanocrystals on the assembly of thick three-dimensional high-performance polypyrrole-based electrodes. J. Energy Chem. 2022, 70, 492–501. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, Y.; Karabulut, E.; Wågberg, L.; Zhu, H.; Hu, L. Nanostructured paper for flexible energy and electronic devices. Mrs Bull. 2013, 38, 320–325. [Google Scholar] [CrossRef]
- Guo, M.; Xu, P.; Lv, J.; Gong, C.; Zhang, Z.; Li, C. Engineering nanocellulose/graphene hybrid aerogel for form-stable composite phase change materials with high phase change enthalpy for energy storage. Diam. Relat. Mater. 2022, 127, 109131. [Google Scholar] [CrossRef]
- Pushparaj, V.L.; Shaijumon, M.M.; Kumar, A.; Murugesan, S.; Ci, L.; Vajtai, R.; Linhardt, R.J.; Nalamasu, O.; Ajayan, P.M. Flexible energy storage devices based on nanocomposite paper. Proc. Natl. Acad. Sci. USA 2007, 104, 13574–13577. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Chiappone, A.; Nair, J.R.; Gerbaldi, C.; Jabbour, L.; Bongiovanni, R.; Zeno, E.; Beneventi, D.; Penazzi, N. Microfibrillated cellulose as reinforcement for Li-ion battery polymer electrolytes with excellent mechanical stability. J. Power Sources 2011, 196, 10280–10288. [Google Scholar] [CrossRef]
- Willgert, M.; Leijonmarck, S.; Lindbergh, G.; Malmström, E.; Johansson, M. Cellulose nanofibril reinforced composite electrolytes for lithium ion battery applications. J. Mater. Chem. A 2014, 2, 13556–13564. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Thielemans, W. Interconnected and high cycling stability polypyrrole supercapacitors using cellulose nanocrystals and commonly used inorganic salts as dopants. J. Energy Chem. 2023, 76, 165–174. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin anode for sodium-ion batteries using natural wood fiber as a mechanical buffer and electrolyte reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef]
- Dinesh, G.; Kandasubramanian, B. Fabrication of transparent paper devices from nanocellulose fiber. Mater. Chem. Phys. 2022, 281, 125707. [Google Scholar] [CrossRef]
- Ko, Y.; Kwon, G.; Choi, H.; Lee, K.; Jeon, Y.; Lee, S.; Kim, J.; You, J. Cutting Edge Use of Conductive Patterns in Nanocellulose-Based Green Electronics. Adv. Funct. Mater. 2023, 2302785. [Google Scholar] [CrossRef]
- Jia, D.; Xie, J.; Dirican, M.; Fang, D.; Yan, C.; Liu, Y.; Li, C.; Cui, M.; Liu, H.; Chen, G. Highly smooth, robust, degradable and cost-effective modified lignin-nanocellulose green composite substrates for flexible and green electronics. Compos. Part B Eng. 2022, 236, 109803. [Google Scholar] [CrossRef]
- Fortunato, E.; Correia, N.; Barquinha, P.; Pereira, L.; Goncalves, G.; Martins, R. High-performance flexible hybrid field-effect transistors based on cellulose fiber paper. IEEE Electron Device Lett. 2008, 29, 988–990. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Yun, S.; Ko, H.-U.; Kim, J. A flexible paper transistor made with aligned single-walled carbon nanotube bonded cellulose composite. Curr. Appl. Phys. 2013, 13, 897–901. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent nanopaper-based flexible organic thin-film transistor array. Adv. Funct. Mater. 2014, 24, 1657–1663. [Google Scholar] [CrossRef]
- Hassinen, T.; Alastalo, A.; Eiroma, K.; Tenhunen, T.-M.; Kunnari, V.; Kaljunen, T.; Forsström, U.; Tammelin, T. All-Printed Transistors on Nano Cellulose Substrate. MRS Adv. 2015, 1, 645–650. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Jimmy, C.Y. Semiconductor/biomolecular composites for solar energy applications. Energy Environ. Sci. 2011, 4, 100–113. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuwawech, K.; Wootthikanokkhan, J.; Tanpichai, S. Enhancement of thermal, mechanical and barrier properties of EVA solar cell encapsulating films by reinforcing with esterified cellulose nanofibres. Polym. Test. 2015, 48, 12–22. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Jiang, Q.; Kacica, C.; Soundappan, T.; Liu, K.-k.; Tadepalli, S.; Biswas, P.; Singamaneni, S. An in situ grown bacterial nanocellulose/graphene oxide composite for flexible supercapacitors. J. Mater. Chem. A 2017, 5, 13976–13982. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, Z.; Liu, D.; Li, Y.; Weadock, N.J.; Fang, Z.; Huang, J.; Hu, L. Biodegradable transparent substrates for flexible organic-light-emitting diodes. Energy Environ. Sci. 2013, 6, 2105–2111. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Nguyen, T.X.; Tang, H.; Zhang, L.; Yang, G. Nano-cellulose 3D-networks as controlled-release drug carriers. J. Mater. Chem. B 2013, 1, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly stretchable piezoresistive graphene–nanocellulose nanopaper for strain sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Johan, M.R.B.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Y.; Fang, R.; Lei, C.; Li, Y.; Li, B.; Pei, Y.; Luo, X. Application of Nanocellulose as particle stabilizer in food Pickering emulsion: Scope, Merits and challenges. Trends Food Sci. Technol. 2021, 110, 573–583. [Google Scholar] [CrossRef]
- Shi, Y.; Jiao, H.; Sun, J.; Lu, X.; Yu, S.; Cheng, L.; Wang, Q.; Liu, H.; Biranje, S.; Wang, J. Functionalization of nanocellulose applied with biological molecules for biomedical application: A review. Carbohydr. Polym. 2022, 285, 119208. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. Recent advances on nanocellulose biomaterials for environmental health photoremediation: An overview. Environ. Res. 2022, 204, 111964. [Google Scholar] [CrossRef] [PubMed]





















| Type of Nanocellulose | Synonyms | Typical Sources | Formation and Average Size |
|---|---|---|---|
| Cellulose nanocrystals (CNCs) | Cellulose nanocrystals, crystallites, whiskers, rod-like cellulose microcrystals | Ramie tunicin, wood, wheat straw, mulberry bark | Method used: acid hydrolysis Ø = 5–70 nm L = 100–250 nm |
| Cellulose nanofibrils (CNFs) | Microfibrillated cellulose, nanofibrils, and microfibrils | Sugar beet, hemp, wood, flax | Mechanical treatment and chemical treatment Ø = 5–60 nm L = several micrometers |
| Bacterial nanocellulose (BNC) | Bacterial cellulose, microbial cellulose, biocellulose | Low-molecular- weight sugar and alcohols | Bacterial-based approach Ø = 20–100 nm |
| Ref. | Raw Materials | Preparation Method | Dimension |
|---|---|---|---|
| [119] | Cladodes of Opuntia Ficus Indica | Homogenization | ~5 nm in width |
| [102] | Sugar beet pulp | TEMPO-mediated oxidation | Not reported |
| [99] | Wheat straw | Cryocrushing and homogenization | 20–120 nm in width |
| [66] | Kraft pulp | Refining and homogenization | 50–100 nm in width |
| [120] | Cotton fibers | Refining | 242 ± 158 nm in diameter |
| [121] | Sugarcane bagasse | Acid hydrolysis | ~32.84 nm |
| [122] | Cotton linter | Ultrasonication | 15–35 nm in diameter |
| [123] | Raw cotton | Acid hydrolysis and alkaline pretreatment | Not reported |
| [124] | Cystoseria myricaas algae | Acid hydrolysis | 10–30 nm |
| [125] | Hibiscus cannabinus | Alkaline pretreatment and acid hydrolysis | Mean diameter of 6.1 ± 5 nm |
| [126] | Imperata brasiliensis grass | Acid hydrolysis | Diameters were 10–60 nm and length 150–250 nm |
| [127] | Amylose maize starch | Electrospinning | 1–4 μm in diameter |
| [128] | Apple and carrot pomaces | Ultrasonication | 3.31–3.54 nm |
| [129] | Peach palm extraction (Bactris gasipaes) | Delignification treatments | Not reported |
| [130] | Moso bamboo culms | Microwave liquefaction and ultrasonication | 567 ± 149 μm in diameter |
| [131] | Areca nut husk | Acid hydrolysis and homogenization | 1–10 nm in diameter |
| [132] | Sugarcane bagasse | Acid hydrolysis | 69–117 nm in length, 6–7 nm in diameter |
| [133] | Oil palm trunk | Acid hydrolysis | 7.67–7.97 nm in diameter, 397–367 nm in length |
| [134] | Banana peel | Alkaline pretreatment and acid hydrolysis | 7.6–10.9 nm in diameter, 454.9–2889.7 nm in length |
| [135] | Raw jute fibers | Alkaline pretreatment and steam explosion | ~50 nm in diameter |
| References | Nanocellulose | Method | Key Findings | Applications |
|---|---|---|---|---|
| [173] | CNCs | H2SO4 hydrolysis | High metal-absorbing capability and good regeneration capacity | Better nanocomposite to remove the contaminant from industrial waste |
| [174] | CNCs | H2SO4 hydrolysis | Improved dispersion and thermodynamic wetting | Reinforcements for hydrophobic materials |
| [151] | Nanocellulose | Noncovalent surface modification | Dispersion ability improved | Thermal energy storage |
| [175] | Nanocellulose | Sulfonation | Improved formation of stable colloidal suspension | Determine aviation energies for the dehydration process |
| [162] | CNCs | Esterification | Cationic charges over the surface of nanocellulose | - |
| [154,176] | CNFs | TEMPO-medicated oxidation | Formation of stable colloidal suspensions | Thermal energy storage |
| [153] | Nanocellulose | Carbonylation | Improves cellulose hydrophobicity | Packing applications |
| [177] | Nanocellulose | Acetylation | Improves cellulose hydrophobicity | Packing applications |
| [178] | CNFs | TEMPO-mediated oxidation | Improved hydrophobicity and thermal stability | Thermal storage |
| Ref. | Class of Nanocellulose | Raw Materials | Special Properties | Field of Application |
|---|---|---|---|---|
| [184] | CNFs | Softwood pulp | High toughness | Nanopaper |
| [282] | CNFs | Not reported | Cell-friendly | 3D bioprinting human chondrocytes |
| [283] | CNFs | Oat straw | High porosity | Selective removal of oil from water |
| [284] | BNC | Not reported | Natural abundance | Energy storage device |
| [285] | CNFs | Bleached softwood pulp | Not reported | Organic light-emitting diodes |
| [231] | CNCs | Not reported | Not reported | Supercapacitor |
| [258] | BNC | Nata de coco (A. xylinum) | Flexible | Organic light-emitting diodes |
| [286] | BNC | Gluconacetobacter xylinum | Not reported | Drug delivery system |
| [287] | CNFs | Not reported | Highly stretchable | Strain sensor |
| [278] | CNFs | Softwood cellulose fibers | Superior optical properties | Conductive paper |
| [288] | CNFs | Not reported | High porosity | Oil absorbent |
| [289] | BNC | Bacteria suspension | Good tensile mechanical properties | Ear cartilage replacement |
| [246] | CNCs | Bleached softwood sulfite pulp | Oblong geometry, lack of cytotoxicity, numerous surface hydroxyl groups | Chemotherapeutic agents against cancer cells |
| [290] | CNCs | Not reported | Ecofriendliness and biodegradability | Antibacterial food packaging |
| [201] | CNFs | Cotton | Not reported | Food packaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofiah, A.G.N.; Pasupuleti, J.; Samykano, M.; Kadirgama, K.; Koh, S.P.; Tiong, S.K.; Pandey, A.K.; Yaw, C.T.; Natarajan, S.K. Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences. Polymers 2023, 15, 3044. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15143044
Sofiah AGN, Pasupuleti J, Samykano M, Kadirgama K, Koh SP, Tiong SK, Pandey AK, Yaw CT, Natarajan SK. Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences. Polymers. 2023; 15(14):3044. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15143044
Chicago/Turabian StyleSofiah, Abd Ghafar Nurhanis, Jagadeesh Pasupuleti, Mahendran Samykano, Kumaran Kadirgama, Siaw Paw Koh, Sieh Kieh Tiong, Adarsh Kumar Pandey, Chong Tak Yaw, and Sendhil Kumar Natarajan. 2023. "Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences" Polymers 15, no. 14: 3044. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15143044