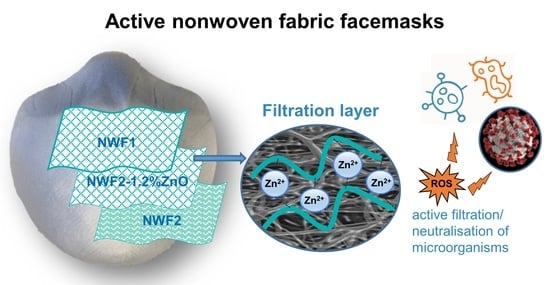
Comparison of Zinc Oxide Nanoparticle Integration into Non-Woven Fabrics Using Different Functionalisation Methods for Prospective Application as Active Facemasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalisation with ZnO NPs
2.3. Non-Woven Fabric Characterisation
2.4. Functionalised Sample Characterisation
2.4.1. SEM-EDS
2.4.2. TEM
2.4.3. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.4. Ground State Diffuse Reflectance (GSDR)
2.4.5. UV-Visible Spectrophotometry (UV-Vis)
2.4.6. Thermogravimetric Analysis (TGA)
2.4.7. Differential Scanning Calorimetry (DSC)
2.4.8. Air and Water Vapour Permeability
2.4.9. Antimicrobial Activity
Antibacterial Activity
2.4.10. Filtration Performance and Breathability
2.4.11. Statistical Analysis
3. Results and Discussion
3.1. NWF Characterisation
3.2. Functionalised NWF Characterization
3.2.1. Morphological and Chemical Analyses
3.2.2. Biological Studies
3.3. Facemask Characterisation
3.3.1. Thermoforming Process
3.3.2. Air Permeability
3.3.3. Breathability and Particle Retention
3.3.4. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control 2016, 44 (Suppl. 9), S102–S108. [Google Scholar] [CrossRef]
- Costa, S.M.; Pacheco, L.; Antunes, W.; Vieira, R.; Bem, N.; Teixeira, P.; Fangueiro, R.; Ferreira, D.P. Antibacterial and Biodegradable Electrospun Filtering Membranes for Facemasks: An Attempt to Reduce Disposable Masks Use. Appl. Sci. 2022, 12, 67. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Pais, V.; Mota, C.; Bessa, J.; Dias, J.G.; Cunha, F.; Fangueiro, R. Study of the Filtration Performance of Multilayer and Multiscale Fibrous Structures. Materials 2021, 14, 7147. [Google Scholar] [CrossRef]
- Laue, M.; Kauter, A.; Hoffmann, T.; Möller, L.; Michel, J.; Nitsche, A. Morphometry of SARS-CoV and SARS-CoV-2 particles in ultrathin plastic sections of infected Vero cell cultures. Sci. Rep. 2021, 11, 3515. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Deng, W.; Sun, Y.; Yao, X.; Subramanian, K.; Ling, C.; Wang, H.; Chopra, S.S.; Xu, B.B.; Wang, J.-X.; Chen, J.-F.; et al. Masks for COVID-19. Adv. Sci. 2022, 9, 2102189. [Google Scholar] [CrossRef]
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.L.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Pan, Z.-M.; Chang, C.-H. Application of a quaternary ammonium agent on surgical face masks before use for pre-decontamination of nosocomial infection-related bioaerosols. Aerosol Sci. Technol. 2016, 50, 199–210. [Google Scholar] [CrossRef]
- Pullangott, G.; Kannan, U.; Gayathri, S.; Kiran, D.V.; Maliyekkal, S.M. A comprehensive review on antimicrobial face masks: An emerging weapon in fighting pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin, N.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Hadinejad, F.; Morad, H.; Jahanshahi, M.; Zarrabi, A.; Pazoki-Toroudi, H.; Mostafavi, E. A Novel Vision of Reinforcing Nanofibrous Masks with Metal Nanoparticles: Antiviral Mechanisms Investigation. Adv. Fiber Mater. 2023, 5, 1273–1317. [Google Scholar] [CrossRef]
- Antunes, J.C.; Moreira, I.P.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Recent Trends in Protective Textiles against Biological Threats: A Focus on Biological Warfare Agents. Polymers 2022, 14, 1599. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Tavakoli, A.; Ataei-Pirkooh, A.; Sadeghi, G.M.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomed. Nanotechnol. Biol. Med. 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012, 96, 363–375. [Google Scholar] [CrossRef]
- Qian, X.; Xiong, S.; Rao, Y.; Low, Z.X.; Zhong, Z.; Wang, Y. Atomic layer deposition of ZnO on polypropylene nonwovens for photocatalytic antibacterial facemasks. Nanotechnology 2023, 34, 255701. [Google Scholar] [CrossRef] [PubMed]
- Benatto, V.G.; de Jesus, J.P.A.; de Castro, A.A.; Assis, L.C.; Ramalho, T.C.; La Porta, F.A. Prospects of ZnS and ZnO as smart semiconductor materials in light-activated antimicrobial coatings for mitigation of severe acute respiratory syndrome coronavirus-2 infection. Mater. Today Commun. 2023, 34, 105192. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Aboubakr, H.A.; Brockgreitens, J.; Hao, W.; Wang, Y.; Goyal, S.M.; Abbas, A. Durable nanocomposite face masks with high particulate filtration and rapid inactivation of coronaviruses. Sci. Rep. 2021, 11, 24318. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, N.K.; El-Sayed, M.; Al-Mofty, S.E.-D.; Mohamed, A.; Karaly, A.H.; El-Naggar, M.E.; Nageh, H.; Sarhan, W.A.; El-Said Azzazy, H.M. Toward Scaling up the Production of Metal Oxide Nanoparticles for Application on Washable Antimicrobial Cotton Fabrics. ACS Omega 2022, 7, 38942–38956. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Manakhov, A.M.; Kiryukhantsev-Korneev, P.V.; Leybo, D.V.; Konopatsky, A.S.; Makarets, Y.A.; Filippovich, S.Y.; Ignatov, S.G.; Shtansky, D.V. Electrospun Polycaprolactone/ZnO Nanocomposite Membranes with High Antipathogen Activity. Polymers 2022, 14, 5364. [Google Scholar] [CrossRef]
- Natsathaporn, P.; Herwig, G.; Altenried, S.; Ren, Q.; Rossi, R.M.; Crespy, D.; Itel, F. Functional Fiber Membranes with Antibacterial Properties for Face Masks. Adv. Fiber Mater. 2023, 5, 1519–1533. [Google Scholar] [CrossRef]
- Belkahla, H.; Antunes, J.C.; Lalatonne, Y.; Sainte Catherine, O.; Illoul, C.; Journé, C.; Jandrot-Perrus, M.; Coradin, T.; Gigoux, V.; Guenin, E.; et al. USPIO–PEG nanoparticles functionalized with a highly specific collagen-binding peptide: A step towards MRI diagnosis of fibrosis. J. Mater. Chem. B 2020, 8, 5515–5528. [Google Scholar] [CrossRef]
- Antunes, J.C.; Ferreira, T.; Bogas, D.; Pais, V.; Bessa, J.; Cunha, F.; Moreira, I.P.; Fangueiro, R. Multilayer and Multiscale Structures for Personal Protective Equipment. Mater. Proc. 2022, 8, 147. [Google Scholar]
- ISO 3801; Textiles—Woven Fabrics—Determination of Mass per Unit Length and Mass per Unit Area. ISO: Geneva, Switzerland, 1977.
- AATCC 195; Test Method for Liquid Moisture Management Properties of Textile Fabrics. AATCC: Research Triangle Park, NC, USA, 2012.
- ASTM-D7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM International: West Conshohocken, PA, USA, 2022.
- Mast, J.; Van Miert, E.; Siciliani, L.; Cheyns, K.; Blaude, M.N.; Wouters, C.; Waegeneers, N.; Bernsen, R.; Vleminckx, C.; Van Loco, J.; et al. Application of silver-based biocides in face masks intended for general use requires regulatory control. Sci. Total Environ. 2023, 870, 161889. [Google Scholar] [CrossRef]
- López-López, J.; Tejeda-Ochoa, A.; López-Beltrán, A.; Herrera-Ramírez, J.; Méndez-Herrera, P. Sunlight Photocatalytic Performance of ZnO Nanoparticles Synthesized by Green Chemistry Using Different Botanical Extracts and Zinc Acetate as a Precursor. Molecules 2022, 27, 6. [Google Scholar] [CrossRef]
- AL-Asady, Z.M.; AL-Hamdani, A.H.; Hussein, M.A. Study the optical and morphology properties of zinc oxide nanoparticles. AIP Conf. Proc. 2020, 2213, 020061. [Google Scholar]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- ISO 11357-1:1997; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. ISO: Geneva, Switzerland, 1997.
- ISO 11357-2:1999; Plastics—Differential Scanning Calorimetry (DSC)—Part 2: Determination of Glass Transition Temperature. ISO: Geneva, Switzerland, 1999.
- ISO 11357-3:1999; Plastics—Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization. ISO: Geneva, Switzerland, 1999.
- ISO 9237:1995; Textiles—Determination of the Permeability of Fabrics to Air. ISO: Geneva, Switzerland, 1995.
- BS 7209:1990; Specification for Water Vapour Permeable Apparel Fabrics. British Standards Institution (BSI): London, UK, 1990.
- BS ISO 22196; Plastics–Measurement of Antibacterial Activity on Plastics Surfaces. ISO: Geneva, Switzerland, 2007.
- JIS Z 2801; Test for Antibacterial Activity and Efficacy. JIS: Tokyo, Japan, 2010.
- ISO 18184; Textiles—Determination of Antiviral Activity of Textile Products. ISO: Geneva, Switzerland, 2014.
- EN ISO 149; Respiratory Protective Devices. Filtering Half Masks to Protect Against Particles—Requirements, Testing, Marking. British Standards Institution (BSI): London, UK, 2009.
- Tsutsumi-Arai, C.; Iwamiya, Y.; Hoshino, R.; Terada-Ito, C.; Sejima, S.; Akutsu-Suyama, K.; Shibayama, M.; Hiroi, Z.; Tokuyama-Toda, R.; Iwamiya, R.; et al. Surface Functionalization of Non-Woven Fabrics Using a Novel Silica-Resin Coating Technology: Antiviral Treatment of Non-Woven Fabric Filters in Surgical Masks. Int. J. Environ. Res. Public Health 2022, 19, 3639. [Google Scholar] [CrossRef] [PubMed]
- Crilley, L.R.; Angelucci, A.A.; Malile, B.; Young, C.J.; VandenBoer, T.C.; Chen, J.I.L. Non-woven materials for cloth-based face masks inserts: Relationship between material properties and sub-micron aerosol filtration. Environ. Sci. Nano 2021, 8, 1603–1613. [Google Scholar] [CrossRef]
- Khan, J.; Momin, S.A.; Mariatti, M.; Vilay, V.; Todo, M. Recent advancements in nonwoven bio-degradable facemasks to ameliorate the post-pandemic environmental impact. Mater. Res. Express 2021, 8, 112001. [Google Scholar] [CrossRef]
- Abd el Mageid, Z.; Ezzat, M.; Elzaki, G.M. Studying the bending stiffness of polyester/linen fabric seams with different structures. Int. J. ChemTech Res. 2016, 9, 1–6. [Google Scholar]
- Lima, M.; Hes, L.; Vasconcelos, R. Frictorq, accessing fabric friction with a novel fabric surface tester. Autex Res. J. 2005, 5, 194–201. [Google Scholar]
- Huang, Y.; Gancheva, T.; Favis, B.D.; Abidli, A.; Wang, J.; Park, C.B. Hydrophobic Porous Polypropylene with Hierarchical Structures for Ultrafast and Highly Selective Oil/Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 16859–16868. [Google Scholar] [CrossRef]
- Adanur, S.; Jayswal, A. Filtration mechanisms and manufacturing methods of face masks: An overview. J. Ind. Text. 2020, 51 (Suppl. 3), 3683S–3717S. [Google Scholar] [CrossRef]
- Özkan, E.T.; Meriç, B. Thermophysiological comfort properties of different knitted fabrics used in cycling clothes. Text. Res. J. 2015, 85, 62–70. [Google Scholar] [CrossRef]
- Onofrei, E.; Rocha, A.M.; Catarino, A. The Influence of Knitted Fabrics’ Structure on the Thermal and Moisture Management Properties. J. Eng. Fibers Fabr. 2011, 6, 155892501100600403. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In Vitro Biocompatibility and Antimicrobial activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. BioNanoScience 2020, 10, 112–121. [Google Scholar]
- Kipnusu, W.K.; Zhuravlev, E.; Schick, C.; Kremer, F. Homogeneous nucleation in polyamide 66, a two-stage process as revealed by combined nanocalorimetry and IR spectroscopy. Colloid Polym. Sci. 2022, 300, 1247–1255. [Google Scholar] [CrossRef]
- Buček, A.; Popelka, A.; Zahoranová, A.; Kovacik, D.; Novak, I.; Černák, M. Acrylic Acid Plasma Treatment of Polypropylene Nonwoven Fabric. Fibres Text. East. Eur. 2016, 24, 161–164. [Google Scholar] [CrossRef]
- Pereira, P.; Ferreira, D.P.; Araújo, J.C.; Ferreira, A.; Fangueiro, R. The Potential of Graphene Nanoplatelets in the Development of Smart and Multifunctional Ecocomposites. Polymers 2020, 12, 2189. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpiłowski, K.; Gun’ko, V.M.; Zarko, V.I. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3-SiO2-TiO2). J. Nanoparticle Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Park, Y.; Cho, H. Improvement in the dispersion stability of iron oxide nanoparticles in highly concentrated brine solution using encapsulation with polymer-polymer crosslinked shells. Adv. Powder Technol. 2020, 31, 4743–4750. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.; Chen, Z.; Guo, R.; Miao, D.; Peng, L.; Wang, Y.; Shang, S. Enhanced electro-conductivity and multi-shielding performance with copper, stainless steel and titanium coating onto PVA impregnated cotton fabric. J. Mater. Sci. Mater. Electron. 2018, 29, 5624–5633. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Domingues, J.M.; Teixeira, M.O.; Teixeira, M.A.; Freitas, D.; Silva, S.F.D.; Tohidi, S.D.; Fernandes, R.D.V.; Padrão, J.; Zille, A.; Silva, C.; et al. Inhibition of Escherichia Virus MS2, Surrogate of SARS-CoV-2, via Essential Oils-Loaded Electrospun Fibrous Mats: Increasing the Multifunctionality of Antivirus Protection Masks. Pharmaceutics 2022, 14, 303. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Sun, M.; Zhang, X.; Chen, W. Surface modification of polypropylene nonwoven fabrics by grafting of polydopamine. Adv. Polym. Technol. 2018, 37, 3519–3528. [Google Scholar] [CrossRef]
- Han, M.-C.; He, H.-W.; Kong, W.-K.; Dong, K.; Wang, B.-Y.; Yan, X.; Wang, L.-M.; Ning, X. High-performance Electret and Antibacterial Polypropylene Meltblown Nonwoven Materials Doped with Boehmite and ZnO Nanoparticles for Air Filtration. Fibers Polym. 2022, 23, 1947–1955. [Google Scholar] [CrossRef]
- Zu, L.; Li, R.; Jin, L.; Lian, H.; Liu, Y.; Cui, X. Preparation and characterization of polypropylene/silica composite particle with interpenetrating network via hot emulsion sol–gel approach. Prog. Nat. Sci. Mater. Int. 2014, 24, 42–49. [Google Scholar] [CrossRef]
- Tomaszewska, K.; Kałużna-Czaplińska, J.; Jóźwiak, W. Thermal and thermo-catalytic degradation of polyolefins as a simple and efficient method of landfill clearing. Pol. J. Chem. Technol. 2010, 12, 50–57. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation Behavior of Polypropylene during Reprocessing and Its Biocomposites: Thermal and Oxidative Degradation Kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT–MCA in polyamide 6. RSC Adv. 2017, 7, 861–869. [Google Scholar] [CrossRef]
- Pannase, A.M.; Singh, R.K.; Ruj, B.; Gupta, P. Decomposition of polyamide via slow pyrolysis: Effect of heating rate and operating temperature on product yield and composition. J. Anal. Appl. Pyrolysis 2020, 151, 104886. [Google Scholar] [CrossRef]
- Dabrowski, F.; Bourbigot, S.; Delobel, R.; Le Bras, M. Kinetic modelling of the thermal degradation: Of polyamide-6 nanocomposite. Eur. Polym. J. 2000, 36, 273–284. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiang, C.Y.; Wang, H.Z.; Yang, C.C. Two step modification of poly(vinyl alcohol) by UV radiation with 2-hydroxy ethyl methacrylate and sol–gel process for the application of polymer electrolyte membrane. J. Membr. Sci. 2009, 341, 186–194. [Google Scholar] [CrossRef]
- Vrandečić, N.; Erceg, M.; Jakić, M.; Klarić, I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Quadri, T.W.; Olasunkanmi, L.O.; Fayemi, O.E.; Solomon, M.M.; Ebenso, E.E. Zinc Oxide Nanocomposites of Selected Polymers: Synthesis, Characterization, and Corrosion Inhibition Studies on Mild Steel in HCl Solution. ACS Omega 2017, 2, 8421–8437. [Google Scholar] [CrossRef] [PubMed]
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, J.; Wang, S.; Zhang, Z.; Yu, H.; Li, J.; Chen, S. Guanidine-functionalized cotton fabrics for achieving permanent antibacterial activity without compromising their physicochemical properties and cytocompatibility. Cellulose 2020, 27, 6027–6036. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, P.; Chen, W. Nano TiO2/amino-benzotriazole functionalization of cotton knitted fabrics for ultraviolet protection and antibacteria by pad-dry method. Text. Res. J. 2023, 93, 80–91. [Google Scholar] [CrossRef]
- Silva, J.; Mesquita, R.; Pinho, E.; Caldas, A.; Oliveira, M.R.; Lopes, C.M.; Lúcio, M.; Soares, G. Incorporation of lipid nanosystems containing omega-3 fatty acids and resveratrol in textile substrates for wound healing and anti-inflammatory applications. SN Appl. Sci. 2019, 1, 1007. [Google Scholar] [CrossRef]








| Studied Parameter | Result Output | NWF1 | NWF2 |
|---|---|---|---|
| Structural | Thickness (mm) | 0.76 ± 0.03 | 0.55 ± 0.02 |
| Areal mass density (g/m2) | 92.46 ± 3.41 | 56.02 ± 1.74 | |
| Physical | Water contact angle (°) | 125.36 ± 11.91 | 127.84 ± 5.47 |
| Mechanical | Bending stiffness (mg.cm) | 3184.99 ± 0.29 | 708.69 ± 0.25 |
| Static µ | 0.25 ± 0.018 | 0.27 ± 0.023 | |
| Kinetic µ | 0.19 ± 0.009 | 0.21 ± 0.006 | |
| Thermal and moisture management | One-way transport capacity, OWTC (%) | 862.35 ± 42.02 | 881.43 ± 126.58 |
| Overall moisture management capacity, OMMC | 0.67 ± 0.02 | 0.73 ± 0.09 | |
| Thermal conductivity λ (10−3 W/m K) | 31.27 ± 0.31 | 28.83 ± 0.40 | |
| Thermal diffusion α (10−6 m2/s) | 0.126 ± 0.021 | 0.179 ± 0.025 | |
| Thermal absorptivity b (Ws1/2/m K) | 89 ± 8 | 69 ± 6 | |
| Thermal resistance R (10−3 m2 K/W) | 24.17 ± 0.97 | 19.10 ± 0.70 | |
| Thermal diffusion P (10−6 m2/s) | 2.08 ± 0.13 | 1.49 ± 0.06 |
| NWF1 | NWF2 | NWF1- NWF2(dip-pad-dry)-NWF2 | NWF1- NWF2(exhaustion)- NWF2 | NWF1- NWF2(electrospinning)-NWF2 | |
|---|---|---|---|---|---|
| PressureInhalation (mbar) | 0.679 | 1.104 | 0.175 | 1.935 | 1.992 |
| PressureExhalation (mbar) | 0.879 | 0.864 | 0.531 | 1.307 | 1.226 |
| Particles penetration (%) | 4.142 | 4.479 | 0.030 | 0.000 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.; Vale, A.C.; Pinto, A.C.; Costa, R.V.; Pais, V.; Sousa, D.; Gomes, F.; Pinto, G.; Dias, J.G.; Moreira, I.P.; et al. Comparison of Zinc Oxide Nanoparticle Integration into Non-Woven Fabrics Using Different Functionalisation Methods for Prospective Application as Active Facemasks. Polymers 2023, 15, 3499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173499
Ferreira T, Vale AC, Pinto AC, Costa RV, Pais V, Sousa D, Gomes F, Pinto G, Dias JG, Moreira IP, et al. Comparison of Zinc Oxide Nanoparticle Integration into Non-Woven Fabrics Using Different Functionalisation Methods for Prospective Application as Active Facemasks. Polymers. 2023; 15(17):3499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173499
Chicago/Turabian StyleFerreira, Tânia, Ana Catarina Vale, Alexandra C. Pinto, Rita V. Costa, Vânia Pais, Diana Sousa, Fernanda Gomes, Graça Pinto, José Guilherme Dias, Inês P. Moreira, and et al. 2023. "Comparison of Zinc Oxide Nanoparticle Integration into Non-Woven Fabrics Using Different Functionalisation Methods for Prospective Application as Active Facemasks" Polymers 15, no. 17: 3499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173499