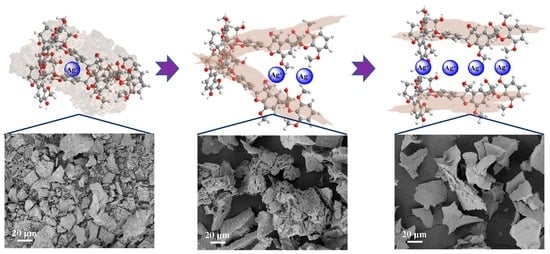
Silver Ions Drive Ordered Self-Assembly Mechanisms and Inherent Properties of Lignin Nanoflowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carboxymethylation Modification of Lignin
2.3. Characterization of Carboxymethylated Lignin
2.4. Aggregation Morphology of Carboxymethylated Lignin in AgNO3 Solution
2.5. Adsorption Kinetics and Thermodynamics of Ag+ on Carboxymethylated Lignin
2.6. Fabrication Process and Performance Analysis of Lignin-Based Layered Nanoflowers
3. Results and Discussion
3.1. Synthesis and Characterization of Carboxymethylated Lignin
3.2. Aggregation Behavior of Carboxymethylated Lignin in AgNO3 Solution
3.3. Adsorption Kinetics of Ag+ on Carboxymethylated Lignin
3.4. Adsorption Thermodynamics of Ag+ on Carboxymethylated Lignin
3.5. Stability of Lignin-Based Layered Nanoflower-Stabilized Pickering Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikulčić, H.; Baleta, J.; Zhang, Z.; Klemeš, J.J. Sustainable development of energy, water and environmental systems in the changing world. J. Clean. Prod. 2023, 390, 135945. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.; Ji, X.; Zhang, F. Fabrication Mechanisms of Lignin Nanoparticles and Their Ultraviolet Protection Ability in PVA Composite Film. Polymers 2022, 14, 4196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, D.; Lin, W.; Wang, H.; Qian, Y.; Qiu, X. In situ synthesis of “brick and mortar”-type lignin-derived carbon/TiO2 composite with a remarkable photocatalytic performance. J. Ind. Eng. Chem. 2021, 97, 216–225. [Google Scholar] [CrossRef]
- Sun, R. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yuan, R.; Wang, D.; Liu, Y.; Chen, F.; Qi, D. Basic Amino Acid-Modified Lignin-Based Biomass Adjuvants: Synthesis, Emulsifying Activity, Ultraviolet Protection, and Controlled Release of Avermectin. Langmuir 2021, 37, 12179–12187. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Wang, G.; Sun, H.; Wang, L.; Liu, Q.; Sui, W.; Parvez, A.M.; Si, C. Lignin Fractionation for Reduced Heterogeneity in Self-Assembly Nanosizing: Toward Targeted Preparation of Uniform Lignin Nanoparticles with Small Size. ACS Sustain. Chem. Eng. 2020, 8, 9174–9183. [Google Scholar] [CrossRef]
- Dorn, L.; Thirion, A.; Ghorbani, M.; Olaechea, L.M.; Mayer, I. Exploring Fully Biobased Adhesives: Sustainable Kraft Lignin and 5-HMF Adhesive for Particleboards. Polymers 2023, 15, 2668. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Huang, P.; Zhong, L.; Gan, S.; Wang, W.; Niu, L. Nitrogen-doped lignin-derived porous carbons for supercapacitors: Effect of nanoporous structure. Chem. Eng. J. 2023, 471, 144817. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Xie, X.; Yang, S.; Sun, L.; Li, T.; Chen, L.; Hua, D. Synthesis of aromatic monomers via hydrogenolysis of lignin over nickel catalyst supported on nitrogen-doped carbon nanotubes. Fuel Process. Technol. 2023, 248, 107810. [Google Scholar] [CrossRef]
- Chen, K.; Lei, L.; Lou, H.; Niu, J.; Yang, D.; Qiu, X.; Qian, Y. High internal phase emulsions stabilized with carboxymethylated lignin for encapsulation and protection of environmental sensitive natural extract. Int. J. Biol. Macromol. 2020, 158, 430–442. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Feng, P.; Huang, P.; Huang, M.; Lin, X.; Feng, Y.; Gan, S.; Han, D.; Wang, W.; et al. Solvent-induced molecular structure engineering of lignin for hierarchically porous carbon: Mechanisms and supercapacitive properties. Ind. Crop. Prod. 2022, 189, 115831. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Yang, D.; Fang, Z.; Liu, W.; Xiang, T.; Qiu, X. Photonic Lignin with Tunable and Stimuli-Responsive Structural Color. ACS Nano 2022, 16, 20705–20713. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, G.; Ge, J.; Wei, N.; Sui, W.; Chen, Z.; Jia, H.; Parvez, A.M.; Si, C. Reduction of lignin heterogeneity for improved catalytic performance of lignin nanosphere supported Pd nanoparticles. Ind. Crop. Prod. 2022, 180, 114685. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Chen, J.; Zhang, D.; Dong, C.; Pang, Z.; Xia, T. Preparation of tailored lignin nanospheres with specific size and tunable structure by self-assembly of lignin from corn straw. Ind. Crop. Prod. 2021, 172, 113993. [Google Scholar] [CrossRef]
- Lo, Y.; Chiu, Y.; Tseng, H.; Chen, J. Thermal-Annealing-Induced Self-Stretching: Fabrication of Anisotropic Polymer Particles on Polymer Films. Langmuir 2017, 33, 12300–12305. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yuan, S.; Li, J.; Zhang, Y.; Chen, F.; Qi, D. Fabrication of flower-like Ag/lignin composites and application in antibacterial fabrics. Int. J. Biol. Macromol. 2022, 222, 783–793. [Google Scholar] [CrossRef]
- Dong, M.; Sun, X.; Bu, T.; Zhang, H.; Wang, J.; He, K.; Li, L.; Li, Z.; Wang, L. 3D/2D TMSs/TiO2 nanofibers heterojunctions for photodynamic-photothermal and oxidase-like synergistic antibacterial therapy co-driven by VIS and NIR biowindows. Compos. B Eng. 2022, 230, 109498. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, R.; Huang, J.; Chen, Z.; Chen, L.; Wu, D.; Fu, R. Construction of 3D carbon networks with well-dispersed SiOx nanodomains from gelable building blocks for lithium-ion batteries. RSC Adv. 2016, 9, 9086–9092. [Google Scholar] [CrossRef]
- Ray, P.G.; Biswas, S.; Roy, T.; Ghosh, S.; Majumder, D.; Basak, P.; Roy, S.; Dhara, S. Sonication assisted hierarchical decoration of Ag-NP on zinc oxide nanoflower impregnated eggshell membrane: Evaluation of antibacterial activity and in vitro cytocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 13717–13733. [Google Scholar] [CrossRef]
- Chen, K.; Qian, Y.; Wang, C.; Yang, D.; Qiu, X.; Binks, B.P. Tumor microenvironment-responsive, high internal phase Pickering emulsions stabilized by lignin/chitosan oligosaccharide particles for synergistic cancer therapy. J. Colloid Interface Sci. 2021, 591, 352–362. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Lai, C.; Xie, Y.; Sun, Y.; Wang, J.; Wang, C.; Yong, Q.; Chu, F.; Zhang, D. Upgrading wood biorefinery: An integration strategy for sugar production and reactive lignin preparation. Ind. Crop. Prod. 2022, 187, 115366. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Lee, Y.; Kim, S.; Son, C.; Cho, K.H.; Lee, S.; Park, Y.; Ren, X.; Chon, K. Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: Enrichment effects of O-containing functional groups. Environ. Res. 2021, 199, 111346. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.B.; Binks, B.P. Catalysis in Pickering emulsions. Soft Matter 2020, 16, 10221–10243. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Desobry-Banon, S.; Gillet, G.; Desobry, S. Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Polymers 2023, 15, 2783. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Y.; Zhu, A.; Zhao, Y.; Wang, H.; Binks, B.P.; Wang, J. Light-Responsive, Reversible Emulsification and Demulsification of Oil-in-Water Pickering Emulsions for Catalysis. Angew. Chem. Int. Ed. 2021, 60, 3928–3933. [Google Scholar] [CrossRef]





| Compound | Grafting Degree (mmol/g) | Mw (Da) | Mn (Da) | PDI | Ph-OH (mmol/g) | -COOH (mmol/g) |
|---|---|---|---|---|---|---|
| EHL | 0 | 3100 | 1100 | 2.82 | 1.55 ± 0.04 | 1.89 ± 0.06 |
| EHL-CM-1 | 0.17 | 3600 | 1900 | 1.89 | 1.38 ± 0.04 | 2.06 ± 0.04 |
| EHL-CM-2 | 0.53 | 4200 | 2200 | 1.90 | 1.02 ± 0.02 | 2.42 ± 0.02 |
| Temperature (°C) | EHL | EHL-CM-1 | EHL-CM-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qem (mg/g) | Kc | R2 | Qem (mg/g) | Kc | R2 | Qem (mg/g) | Kc | R2 | |
| 60 °C | 1046.0 | 73,593 | 0.9983 | 1157.5 | 108,779 | 0.9996 | 1234.5 | 244,588 | 0.9997 |
| 70 °C | 1403.3 | 81,530 | 0.9316 | 1464.9 | 179,089 | 0.9997 | 2186.2 | 561,327 | 0.9996 |
| 80 °C | 1811.8 | 99,567 | 0.9997 | 2039.5 | 287,606 | 0.9999 | 3074.9 | 3,256,851 | 0.9999 |
| T (K) | EHL | EHL-CM-1 | EHL-CM-2 |
|---|---|---|---|
| ΔG (kJ/mol) | −32.3 ± 1.1 | −34.5 ± 2.0 | −38.7 ± 4.0 |
| ΔH (kJ/mol) | 14.7 | 47.5 | 125.5 |
| ΔS (J/(mol·K)) | 173.1 | 238.8 | 478.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Liu, E.; Yuan, S.; Zhang, B. Silver Ions Drive Ordered Self-Assembly Mechanisms and Inherent Properties of Lignin Nanoflowers. Polymers 2023, 15, 3541. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173541
Chen K, Liu E, Yuan S, Zhang B. Silver Ions Drive Ordered Self-Assembly Mechanisms and Inherent Properties of Lignin Nanoflowers. Polymers. 2023; 15(17):3541. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173541
Chicago/Turabian StyleChen, Kai, Encheng Liu, Shengrong Yuan, and Baoquan Zhang. 2023. "Silver Ions Drive Ordered Self-Assembly Mechanisms and Inherent Properties of Lignin Nanoflowers" Polymers 15, no. 17: 3541. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15173541