Ring-Opening Polymerization of rac-β-Butyrolactone Promoted by New Tetradentate Thioether-Amide Ligand-Type Zinc Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
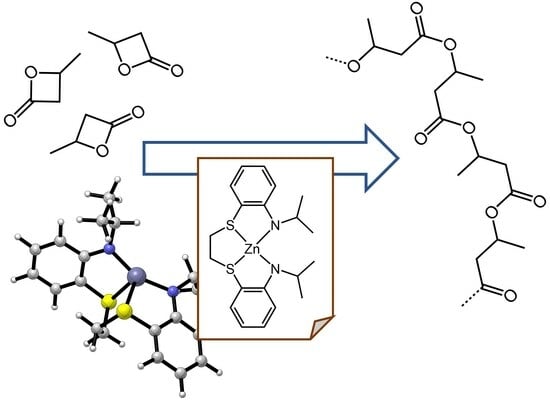
2.2. Polymerization Studies
2.3. Mechanism of β-BL Polymerization by Zinc Complexes
3. Conclusions
4. Experimental Section
4.1. Synthesis of (NSSN-iPr)Zn (1)
4.2. Synthesis of (NSSN-Cy)Zn (2)
4.3. Synthesis of (NSSN-Mes)Zn (3)
4.4. Typical Procedure for Cyclic Ester Polymerization
4.5. Computational Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rieger, B.; Künkel, A.; Coates, G.W.; Reichardt, R.; Dinjus, E.; Zevaco, T.A. Synthetic Biodegradable Polymers; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.-Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Muhammadi, S.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Li, H.; Shakaroun, R.M.; Guillaume, S.M.; Carpentier, J.F. Recent Advances in Metal-Mediated Stereoselective Ring-Opening Polymerization of Functional Cyclic Esters towards Well-Defined Poly(hydroxy acid)s: From Stereoselectivity to Sequence-Control. Chem.Eur. J. 2020, 26, 128–138. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Cheng, M.; Attygalle, A.B.; Lobkovsky, E.B.; Coates, G.W. Single-Site Catalysts for Ring-Opening Polymerization: Synthesis of Heterotactic Poly(lactic acid) from rac-Lactide. J. Am. Chem. Soc. 1999, 121, 11583–11584. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Thevenon, A.; Romain, C.; Bennington, M.S.; White, A.J.P.; Davidson, H.J.; Brooker, S.; Williams, C.K. Dizinc Lactide Polymerization Catalysts: Hyperactivity by Control of Ligand Conformation and Metallic Cooperativity. Angew. Chem. Int. Ed. 2016, 55, 8680–8685. [Google Scholar] [CrossRef]
- D’Auria, I.; Lamberti, M.; Mazzeo, M.; Milione, S.; Roviello, G.; Pellecchia, C. Coordination Chemistry and Reactivity of Zinc Complexes Supported by a Phosphido Pincer Ligand. Chem.-Eur. J. 2012, 18, 2349–2360. [Google Scholar] [CrossRef]
- Pilone, A.; Lamberti, M.; Mazzeo, M.; Milione, S.; Pellecchia, C. Ring-Opening Polymerization of Cyclic Esters by Phenoxy-Thioether Complexes Derived from Biocompatible Metals. Dalton Trans. 2013, 42, 13036–13047. [Google Scholar] [CrossRef]
- Rieth, L.R.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Single-site beta-diiminate zinc catalysts for the ring-opening polymerization of beta-butyrolactone and beta-valerolactone to poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 2002, 51, 15239–15248. [Google Scholar] [CrossRef]
- Guillaume, C.; Carpentier, J.-F.; Guillaume, S.M. Immortal ring-opening polymerization of β-butyrolactone with zinc catalysts: Catalytic approach to poly(3-hydroxyalkanoate). Polymer 2009, 50, 5909–5917. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, C.H.; Ko, B.T.; Ho, R.M. Ring-opening polymerization of β-butyrolactone catalyzed by efficient magnesium and zinc complexes derived from tridentate anilido-aldimine ligand. J. Polym. Sci. A Polym. Chem. 2010, 48, 5339–5347. [Google Scholar] [CrossRef]
- Le Borgne, A.; Spassky, N. Stereoelective polymerization of β-butyrolactone. Polymer 1989, 30, 2312–2319. [Google Scholar] [CrossRef]
- Kronast, A.; Reiter, M.; Altenbuchner, P.T.; Jandl, C.; Pöhig, A.; Rieger, B. Electron-Deficient β-Diiminato-Zinc-Ethyl Complexes: Synthesis, Structure, and Reactivity in Ring-Opening Polymerization of Lactones. Organometallics 2016, 35, 681–685. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Aluthge, D.C.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Highly Active Chiral Zinc Catalysts for Immortal Polymerization of β-Butyrolactone Form Melt Processable Syndio-Rich Poly(hydroxybutyrate). Macromolecules 2016, 23, 8812–8824. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiterm, M.; Bucalon, D.H.; Altmann, P.J.; Kronast, A.; Rieger, B. Synthesis of Lewis Acidic, Aromatic Aminotroponiminate Zinc Complexes for the Ring-Opening Polymerization of Cyclic Esters. Inorg. Chem. 2018, 57, 9931–9940. [Google Scholar] [CrossRef]
- Shaik, M.; Peterson, J.; Du, G. Cyclic and linear polyhydroxylbutyrates from ring-opening polymerization of β-butyrolactone with amido-oxazolinate zinc catalysts. Macromolecules 2019, 52, 157–166. [Google Scholar] [CrossRef]
- Gruszka, W.; Walker, L.C.; Shaver, M.P.; Garden, J.A. In Situ Versus Isolated Zinc Catalysts in the Selective Synthesis of Homo and Multi-block Polyesters. Macromolecules 2020, 53, 4294–4302. [Google Scholar] [CrossRef]
- Luciano, E.; Buonerba, A.; Grassi, A.; Milione, S.; Capacchione, C. Thioetherphenolate Group 4 Metal Complexes in the Ring Opening Polymerization of rac-β-Butyrolactone. J. Polym. Sci. Part A 2016, 54, 3132–3139. [Google Scholar] [CrossRef]
- Lapenta, R.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Milione, S.; Capacchione, C. Stereorigid OSSO-type Group 4 Metal Complexes in the Ring Opening Polymerization of rac-Lactide. Inorg. Chem. 2017, 56, 3447–3458. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Mosquera, M.E.G.; Cano, J.; Milione, S. Biodegradable PHB from rac-b-Butyrolactone: Highly Controlled ROP Mediated by a Penta-Coordinated Aluminum Complex. Organometallics 2018, 37, 837–840. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Cuenca, T.; Mosquera, M.E.G.; Milione, S.; Cano, J. Ring-Opening Polymerization (ROP) of Cyclic Esters by a Versatile Aluminum Diphenoxyimine Complex: From Polylactide to Random Copolymers. Eur. Polym. J. 2020, 125, 109527. [Google Scholar] [CrossRef]
- Impemba, S.; Roviello, G.; Milione, S.; Capacchione, C. NSSN-Type Group 4 Metal Complexes in the Ring-Opening Polymerization of l-Lactide. Inorg. Chem. 2021, 60, 7561–7572. [Google Scholar] [CrossRef] [PubMed]
- Impemba, S.; Tozio, I.; Roviello, G.; Mameri, S.; Dagone, S.; Milione, S. Mono- and Bimetallic Aluminum Complexes Supported by Thioether-Amide Ligands: Synthesis, Characterization, and Application in the Ring Opening Polymerization of l-Lactide. Organometallics 2023, 42, 921–932. [Google Scholar] [CrossRef]
- Ritacco, I.; Voccia, M.; Impemba, S.; Camellone, M.F.; Milione, S.; Caporaso, L. Electronic and Steric Effects on L-Lactide Ring-Opening Polymerization with NSSN-type Zr(IV) Complexes. Eur. J. Inorg. Chem. 2023, 26, e202200588. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical Hybrid Density Functional with Perturbative Second-Order Correlation. J. Chem. Phys. 2006, 124, 034108. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Fox, Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cioslowski, J. A New Population Analysis Based on Atomic Polar Tensors. J. Am. Chem. Soc. 1989, 111, 8333–8336. [Google Scholar] [CrossRef]








| Complex | Zn Charge (a) | %VBur (b) | Zn-N (c) | S-Zn-N (d) |
|---|---|---|---|---|
| 1 | 1.39 | 74.3 | 1.94 | 86.1 |
| 2 | 1.39 | 78.4 | 1.93 | 86.0 |
| 3 | 1.23 | 83.2 | 1.93 | 85.2 |
| Entry (a) | Catalyst | Time (min) | Conv. (b) (%) | TOF (c) (h−1) | Mn(th) (d) | Mn(expt) (e) | PDI (e) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 30 | 84 | 168 | 7.2 | 7.2 | 1.17 |
| 2 | 2 | 30 | 77 | 154 | 6.6 | 6.0 | 1.13 |
| 3 | 3 | 30 | 64 | 128 | 5.5 | 5.2 | 1.21 |
| 4 | 1 | 60 | 97 | 97 | 8.3 | 8.2 | 1.22 |
| 5 | 2 | 60 | 90 | 90 | 7.7 | 7.5 | 1.23 |
| 6 | 3 | 60 | 90 | 90 | 7.7 | 7.3 | 1.91 |
| Entry (a) | Monomer | [mon]/[1] | Time (min) | Conv. (c) (%) | TOF (d) (h−1) | Mn(th) (e) | Mn(expt) (f) | PDI (f) |
|---|---|---|---|---|---|---|---|---|
| LLA | 100 | 30 | 90 | 180 | 13.0 | 11.9 | 1.19 | |
| 7 (b) | LLA | 250 | 120 | 95 | 406 | 34.2 | 30.3 | 1.25 |
| 8 | εCL | 100 | 5 | 99 | 1200 | 11.3 | 9.8 | 1.15 |
| 9 | εCL | 300 | 10 | 99 | 1930 | 33.8 | 31.1 | 1.28 |
| 10 | εCL | 600 | 15 | 99 | 2600 | 67.7 | 62.5 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Impemba, S.; Manca, G.; Tozio, I.; Milione, S. Ring-Opening Polymerization of rac-β-Butyrolactone Promoted by New Tetradentate Thioether-Amide Ligand-Type Zinc Complexes. Polymers 2023, 15, 4366. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15224366
Impemba S, Manca G, Tozio I, Milione S. Ring-Opening Polymerization of rac-β-Butyrolactone Promoted by New Tetradentate Thioether-Amide Ligand-Type Zinc Complexes. Polymers. 2023; 15(22):4366. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15224366
Chicago/Turabian StyleImpemba, Salvatore, Gabriele Manca, Irene Tozio, and Stefano Milione. 2023. "Ring-Opening Polymerization of rac-β-Butyrolactone Promoted by New Tetradentate Thioether-Amide Ligand-Type Zinc Complexes" Polymers 15, no. 22: 4366. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15224366