Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
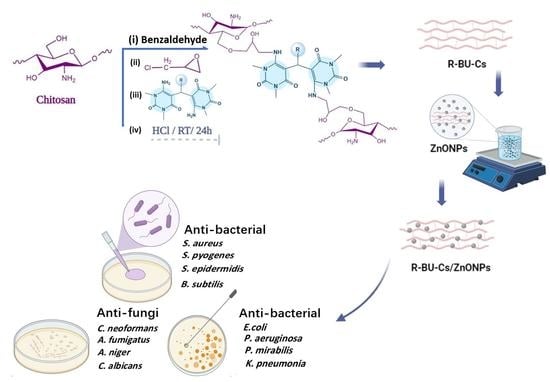
2.2.1. Synthesis of Substituted Bisuracil (R-BU) Derivatives
2.2.2. Synthesis of Novel R-BU-CsSB and R-BU-Cs Hydrogels
2.2.3. Synthesis of 2Nph-BU-Cs/ZnONPs and 2Mph-BU-Cs/ZnONPs Composites
2.3. Measurements
2.3.1. Elemental Analysis
2.3.2. FTIR Spectroscopy
2.3.3. X-ray Diffractometry
2.3.4. Scanning Electron Microscopy
2.3.5. EDS Measurements
2.3.6. Determination of the Minimal Inhibitory Concentration (MIC) Using XTT Assay
2.3.7. Cytotoxicity Evaluation Using Viability Assay
3. Results and Discussion
3.1. Synthesis of R-BU-CsSB and R-BU-Cs Hydrogels and ZnONPs Bio-Composites
3.2. Characterization of R-BU-CsSB and R-BU-Cs Hydrogels and ZnONPs Bio-Composites
3.2.1. Elemental Analysis
3.2.2. FTIR Spectroscopy
3.2.3. Powder X-ray Diffractometry (XRD)
3.2.4. SEM Analysis
3.2.5. Energy-Dispersive Spectroscopy (EDS)
3.3. Antibacterial Activity
3.4. Antifungal Activity
3.5. Cytotoxicity Evaluation
4. Conclusions
- Various substituted bisuracil (R-BU) derivatives are efficient crosslinkers for binding chitosan Schiff’s base chains (R-BU-CsSB) and chitosan chains (R-BU-Cs).
- A simple and easy multi-step method was utilized for chemical cross-linking processes; first, by a condensation of the bioactive 1ry amino groups of chitosan with benzaldehyde molecules to yield CsSB and thus protecting them from reaction with epichlorohydrin; second, by a selective reaction between the 1ry hydroxyl groups at C6 of the obtained CsSB and epichlorohydrin to produce ECsSB; third, by a facile opening of the epoxy rings of the latter via a reaction with the amino groups of R-BU derivatives to obtain crosslinked polymeric matrices containing additional bioactive nitrogen-rich BU moieties (R-BU-CsSB hydrogels); and fourth, by elimination of the benzaldehyde moieties of the latter to retrieve the bioactive amino groups on chitosan, producing R-BU-Cs hydrogels.
- The more reactive and biologically active amino groups on chitosan did not consume because the modifications were confined on 1ry -OH groups at C6 on chitosan.
- Dispersion of three various amounts of ZnONPs (1, 3, and 5% based on the hydrogel weight) inside 2Nph-BU-Cs and 2Mph-BU-Cs hydrogels led to the formation of six ZnONPs bio-composites. Several proper analytical methods proved the successful formation of these composites.
- The inhibition potency of R-BU-CsSB and R-BU-Cs hydrogels as well as the ZnONPs bio-composites against all the tested microbes were arranged as: ZnONPs bio-composites > R-BU-Cs hydrogels > R-BU-CsSB hydrogels > Cs.
- Both R-BU-Cs and R-BU-CsSB hydrogels as well as the ZnONPs bio-composites do better in inhibiting Gram-positive bacteria than Gram-negative ones. MIC values were influenced by the type and position of substituents in the aryl ring of R-BU linkages in the hydrogels being lower for the substituents of negative resonance effect (−R) and higher for those of positive resonance effect (+R).
- Compared with the used standard antibiotics, both 2Nph-BU-Cs/ZnONPs and 2Mph-BU-Cs/ZnONPs composites have shown much stronger inhibition activity against most of the inspected Gram-negative bacteria, all the tested Gram-positive ones, and all of the investigated fungi.
- 2Nph-BU-Cs, 2Nph-BU-Cs/ZnONPs-5%, 2Mph-BU-Cs, and 2Mph-BU-Cs/ZnONPs-5% are not hazardous to normal human cells.
- Incorporation of R-BU linkages and ZnONPs into chitosan strengthens its inhibitory action on the growth of hazardous microorganisms. This represents an appropriate technique for obtaining promising compounds that can effectively compete with common antibiotics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Synthesis and characterization of novel trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels modified with multi-walled carbon nanotubes for enhancement of antimicrobial activity. Int. J. Biol. Macromol. 2019, 132, 416–428. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Designing, preparation and evaluation of the antimicrobial activity of biomaterials based on chitosan modified with silver nanoparticles. Int. J. Biol. Macromol. 2020, 151, 92–103. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel CrossLinked O-CM-Chitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Bin Arifin, M.A.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef] [PubMed]
- Alnawmasi, J.S. Construction of amino-thiol functionalized ion-imprinted chitosan for lead (II) ion removal. Carbohydr. Polym. 2023, 308, 120596. [Google Scholar] [CrossRef]
- Bahramzadeh, E.; Yilmaz, E.; Adali, T. Chitosan-graft-poly(N-hydroxy ethyl acrylamide) copolymers: Synthesis, characterization and preliminary blood compatibility in vitro. Int. J. Biol. Macromol. 2018, 123, 1257–1266. [Google Scholar] [CrossRef]
- Hassan, M.M. Enhanced antimicrobial activity and reduced water absorption of chitosan films graft copolymerized with poly(acryloyloxy)ethyltrimethylammonium chloride. Int. J. Biol. Macromol. 2018, 118, 1685–1695. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Elzanaty, A.M.; Abdel-Gawad, O.F.; Arafa, E.G. Synthesis, characterization and antimicrobial activity of Schiff bases modified chitosan-graft-poly(acrylonitrile). Int. J. Biol. Macromol. 2018, 109, 1280–1291. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Abdallah, H.M.; Mohamed, N.A.; Mohamed, R.R. Synthesis, characterization and application of biodegradable crosslinked carboxymethyl chitosan/poly(vinyl alcohol) clay nanocomposites. Mater. Sci. Eng. C 2015, 56, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W. Cytotoxicity and metal ions removal using antibacterial biodegradable hydrogels based on N -quaternized chitosan/poly(acrylic acid). Int. J. Biol. Macromol. 2017, 98, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.R.; Rizk, N.; El Hady, B.M.A.; Abdallah, H.; Sabaa, M.W. Synthesis, Characterization and Application of Biodegradable Crosslinked Carboxymethyl Chitosan/Poly(Ethylene Glycol) Clay Nanocomposites. J. Polym. Environ. 2016, 25, 667–682. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-Mehbad, N.Y. Novel terephthaloyl thiourea cross-linked chitosan hydrogels as antibacterial and antifungal agents. Int. J. Biol. Macromol. 2013, 57, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; El-Ghany, N.A.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018, 115, 651–662. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Ghany, N.A.A. Synthesis, characterization, and antimicrobial activity of chitosan hydrazide derivative. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 410–415. [Google Scholar] [CrossRef]
- Pałasz, A.; Cież, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Zare, A.; Ghobadpoor, A.; Safdari, T. Preparation, characterization and utilization of a novel dicationic molten salt as catalyst for the synthesis of bis(6-amino-1,3-dimethyluracil-5-yl)methanes. Res. Chem. Intermed. 2020, 46, 1319–1327. [Google Scholar] [CrossRef]
- Fathalla, M.; Lawrence, C.M.; Zhang, N.; Sessler, J.L.; Jayawickramarajah, J. Base-pairing mediated non-covalent polymers. Chem. Soc. Rev. 2009, 38, 1608–1620. [Google Scholar] [CrossRef]
- Das, S.; Thakur, A.J. A Clean, Highly Efficient and One-Pot Green Synthesis of Aryl/Alkyl/Heteroaryl-Substituted Bis(6-amino-1,3-dimethyluracil-5-yl)methanes in Water. Eur. J. Org. Chem. 2011, 2011, 2301–2308. [Google Scholar] [CrossRef]
- Bardagí, J.I.; Rossi, R.A. Advances in the Synthesis of 5- and 6-Substituted Uracil Derivatives. Org. Prep. Proced. Int. 2009, 41, 479–514. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Bauza, A.; Banik, R.; Biswas, S.C.; Frontera, A.; Das, S. Structural basis for molecular recognition, theoretical studies and anti-bacterial properties of three bis-uracil derivatives. Tetrahedron 2014, 70, 6931–6937. [Google Scholar] [CrossRef]
- Vijayakumar, B.G.; Ramesh, D.; Manikandan, K.S.; Theresa, M.; Sethumadhavan, A.; Priyadarisini, V.B.; Radhakrishnan, E.K.; Mani, M.; Kannan, T. Chitosan with pendant (E)-5-((4-acetylphenyl)diazenyl)-6-aminouracil groups as synergetic antimicrobial agents. J. Mater. Chem. B 2022, 10, 4048–4058. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Synthesis and Characterization of Novel Uracil-Modified Chitosan as a Promising Adsorbent for Efficient Removal of Congo Red Dye. Polymers 2022, 14, 271. [Google Scholar] [CrossRef]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Abiraman, T.; Kavitha, G.; Rengasamy, R.; Balasubramanian, S. Antifouling behavior of chitosan adorned zinc oxide nanorods. RSC Adv. 2016, 6, 69206–69217. [Google Scholar] [CrossRef]
- Dananjaya, S.; Kumar, R.S.; Yang, M.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int. J. Biol. Macromol. 2018, 108, 1281–1288. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.-J. Chitosan-Zinc Oxide hybrid composite for enhanced dye degradation and antibacterial activity. Compos. Interfaces 2013, 20, 365–377. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Terephthalohydrazido cross-linked chitosan hydrogels: Synthesis, characterization and applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 969–982. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A.; El-Ghany, N.A.A. Evaluation of the antimicrobial and anti-biofilm activity of novel salicylhydrazido chitosan derivatives impregnated with titanium dioxide nanoparticles. Int. J. Biol. Macromol. 2022, 205, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, F.; Storey, D.G.; Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, T.F.; Storey, D.G. Rapid Colorimetric Assay for Antimicrobial Susceptibility Testing of Pseudomonas Aeruginosa Rapid Colorimetric Assay for Antimicrobial Susceptibility Testing of Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2004, 48, 1879–1881. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of Antimicrobial and Anti-Biofilm Formation Activities of Novel Poly(vinyl alcohol) Hydrogels Reinforced with Crosslinked Chitosan and Silver Nano-Particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of Chitosan/Zinc Oxide Nanoparticles Stabilized by Chitosan via Microwave Heating. Bull. Chem. React. Eng. Catal. 2019, 14, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.; Hu, Y.; Shi, L.; Chen, J.; Cui, A.; Weng, W.; Osako, K. Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci. Rep. 2021, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bashal, A.H.; Riyadh, S.M.; Alharbi, W.; Alharbi, K.H.; Farghaly, T.A.; Khalil, K.D. Bio-Based (Chitosan-ZnO) Nanocomposite: Synthesis, Characterization, and Its Use as Recyclable, Ecofriendly Biocatalyst for Synthesis of Thiazoles Tethered Azo Groups. Polymers 2022, 14, 386. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2018, 124, 1132–1136. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Pandey, A.; Verma, S.P.; Das, P.; Tewari, R. Efficient water soluble nanostructured ZnO grafted O-carboxymethyl chitosan/curcumin-nanocomposite for cancer therapy. Process Biochem. 2015, 50, 678–688. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Pandey, A.; Verma, S.P.; Das, P.; Tewari, R.P. In situ grafted nanostructured ZnO/carboxymethyl cellulose nanocomposites for efficient delivery of curcumin to cancer. J. Polym. Res. 2014, 21, 550. [Google Scholar] [CrossRef]
- Kucharska, M.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 169–187. ISBN 9781119450467. [Google Scholar]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W. Chitosan Both Activates Genes in Plants and Inhibits RNA Synthesis in Fungi. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Springer: Boston, MA, USA, 1986; pp. 209–214. ISBN 978-1-4613-2167-5. [Google Scholar]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sarkar, P. Understanding the interaction of DNA–RNA nucleobases with different ZnO nanomaterials. Phys. Chem. Chem. Phys. 2014, 16, 15355–15366. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W.; Muzzarelli, R.A.A.; Jeuniaux, C.; Gooday, C. Chitin in Nature and Technology; Springer: New York, NY, USA, 1986; Volume 467, pp. 209–214. [Google Scholar]
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles–friends or enemies? Int. J. Environ. Health Res. 2020, 32, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Eweis, M.; Elkholy, S.; Elsabee, M. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int. J. Biol. Macromol. 2006, 38, 1–8. [Google Scholar] [CrossRef]

















| Sample Code | Elemental Analysis | ||||
|---|---|---|---|---|---|
| %C | %H | %N | %O | %Cl | |
| Cs | 44.90 | 6.86 | 8.61 | 39.63 | - |
| CsSB | 62.80 | 5.98 | 5.70 | 25.52 | - |
| ECsSB | 63.08 | 6.11 | 4.48 | 26.33 | - |
| 2Nph-BU-CsSB | 58.30 | 5.63 | 11.89 | 24.18 | - |
| 2Nph-BU-Cs | 50.56 | 5.79 | 14.47 | 29.18 | - |
| 2Clph-BU-CsSB | 58.62 | 5.65 | 10.79 | 21.52 | 3.42 |
| 2Clph-BU-Cs | 51.14 | 5.83 | 13.00 | 25.80 | 4.23 |
| 4Clph-BU-CsSB | 58.55 | 5.68 | 10.78 | 21.54 | 3.45 |
| 4Clph-BU-Cs | 51.09 | 5.80 | 12.98 | 25.97 | 4.16 |
| Ph-BU-CsSB | 60.80 | 5.94 | 11.09 | 22.17 | - |
| Ph-BU-Cs | 53.45 | 6.24 | 13.46 | 26.85 | - |
| 2Mph-BU-CsSB | 59.39 | 6.38 | 10.90 | 23.33 | - |
| 2Mph-BU-Cs | 53.11 | 6.19 | 12.92 | 27.78 | - |
| H-BU-CsSB | 57.79 | 6.08 | 12.10 | 24.03 | - |
| H-BU-Cs | 49.09 | 6.35 | 14.85 | 29.71 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity. Polymers 2023, 15, 980. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15040980
Alharbi RA, Alminderej FM, Al-Harby NF, Elmehbad NY, Mohamed NA. Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity. Polymers. 2023; 15(4):980. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15040980
Chicago/Turabian StyleAlharbi, Rana A., Fahad M. Alminderej, Nouf F. Al-Harby, Noura Y. Elmehbad, and Nadia A. Mohamed. 2023. "Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity" Polymers 15, no. 4: 980. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15040980