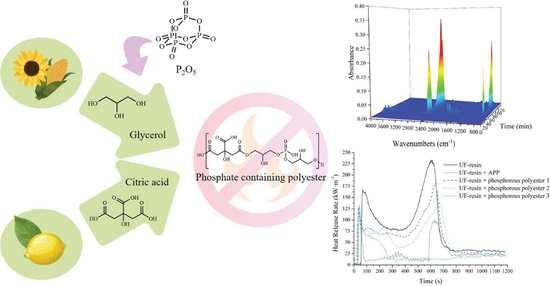
Bio-Based Phosphate-Containing Polyester for Improvement of Fire Reaction in Wooden Particleboard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Phosphate Containing Polyester
2.3. Particleboard Formation
2.4. Measurements
2.4.1. ATR-FTIR Characterization
2.4.2. 1H-NMR Characterization
2.4.3. SEM-EDX
2.4.4. TGA Analysis
2.4.5. TGA-FTIR Analysis
2.4.6. Water Swelling Performance and Density Determination
2.4.7. Cone Calorimeter Tests
3. Results and Discussion
3.1. Phosphorylated Polyester
3.1.1. ATR-FTIR Characterization
3.1.2. 1H-NMR Characterization
3.1.3. SEM-EDX
3.1.4. TGA Analysis
3.1.5. TGA-FTIR Analysis
3.2. Particle Board Performances
3.2.1. Water Swelling and Absorption
3.2.2. Cone Calorimeter Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Thygesen, L.; Marlair, G.; Angelici, C.; De Jong, E.; Sbirrazzuoli, N. Humin based resin for wood modification and property improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef] [Green Version]
- Pin, J.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; Van Der Waal, J.; De Jong, E. Valorization of biorefinery side-stream products: Combination of humins with polyfurfuryl alcohol for composite elaboration. ACS Sustain. Chem. Eng. 2014, 2, 2182–2190. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Peng, Y.; Wang, W.; Cao, J. Thermal behavior and flame retardancy of poplar wood impregnated with furfuryl alcohol catalyzed by boron/phosphorus compound system. Ind. Crops Prod. 2022, 176, 114361. [Google Scholar] [CrossRef]
- Richter, F.; Jervis, F.; Huang, X.; Rein, G. Effect of oxygen on the burning rate of wood. Combust. Flame. 2021, 234, 111591. [Google Scholar] [CrossRef]
- Owodunni, A.; Lamaming, J.; Hashim, R.; Taiwo, O.; Hussin, M.; Kassim, M.M.; Bustami, Y.; Sulaiman, O.; Amini, M.; Hiziroglu, S. Adhesive application on particleboard from natural fibers: A review. Polym. Compos. 2020, 41, 4448–4460. [Google Scholar] [CrossRef]
- Lee, S.; Lum, W.; Boon, J.; Kristak, L.; Antov, P.; Pędzik, M.; Rogoziński, T.; Taghiyari, H.; Lubis, M.; Fatriasari, W.; et al. Particleboard from agricultural biomass and recycled wood waste: A review. J. Mater. Res. Technol. 2022, 20, 4630–4658. [Google Scholar] [CrossRef]
- Owodunni, A.; Hashim, R.; Taiwo, O.; Hussin, M.; Kassim, M.; Bustami, Y.; Sulaiman, O.; Amini, M.; Hiziroglu, S. Flame-retardant Properties of Particleboard Made from Coconut Fibre Using Modified Potato Starch as a Binder. J. Phys. Sci. 2020, 31, 129–143. [Google Scholar] [CrossRef]
- Odozi, T.; Akaranta, O.; Ejike, P. Wood-based, Particle Boards from Agricultural Wastes. Agric. Wastes 1986, 16, 237–240. [Google Scholar] [CrossRef]
- Yue, K.; Liang, B.; Liu, J.; Li, M.; Pu, Y.; Lu, W.; Han, Z.; Li, Z. Fire resistance of light wood frame walls sheathed with innovative gypsum-particle composite: Experimental investigations. J. Build. Eng. 2022, 45, 103576. [Google Scholar] [CrossRef]
- Medved, S.; Jones, D.; Faelled, P.; Pirs, D.; Humar, M.; Lesar, B. Investigation of fire-retardant additive on particleboard proerties. Proc. Int. Panel Prod. Symp. 2019, 2019, 141–148. [Google Scholar]
- Hakkarainen, T.; Mikkola, E.; Östman, B.; Tsantaridis, L.; Brumer, H.; Piispanen, P. Innovative Eco-efficient High Fire Performance Wood Products for Demanding Applications: State-of-the-Art; SP Rapport: Stockholm, Sweden, 2005. [Google Scholar]
- COMMISSION REGULATION (EC) No 552/2009 of 22 June 2009 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annex XVII. Off. J. Eur. Union 2009, 164, 7–31. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:164:0007:0031:EN:PDF (accessed on 9 May 2020).
- DIRECTIVE 2002/95/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off. J. Eur. Union 2003, 37, 19–23. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:037:0019:0023:EN:PDF (accessed on 9 May 2020).
- Illy, N.; Fache, M.; Ménard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of bio-based compounds: State of the art. Polym. Chem. 2015, 6, 6257–6291. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Guan, J.; Tang, R.; Liu, K. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Qu, X.; Zhou, G. Poly(hexamethylene 2,5-furandicarboxylate) copolyesters containing phosphorus: Synthesis, crystallization behavior, thermal, mechanical and flame retardant properties. Polym. Degrad. Stab. 2018, 153, 272–280. [Google Scholar] [CrossRef]
- Chang, B.; Thakur, S.; Mohanty, A.; Misra, M. Novel sustainable biobased flame retardant from functionalized vegetable oil for enhanced flame retardancy of engineering plastic. Sci. Rep. 2019, 9, 15971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Liu, M.; Deng, D.; Zhao, R.; Liu, X.; Yang, Y.; Yang, S.; Liu, X. Phosphorus-containing soybean oil-derived polyols for flame-retardant and smoke-suppressant rigid polyurethane foams. Polym. Degrad. Stab. 2021, 191, 109701. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.; Chen, J.; Mishra, S.; Gupta, R. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 16–19. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell, C.; Fache, M.; Ferry, L.; Sonnier, R.; David, G. From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Howell, B.; Daniel, Y. Incorporation of comonomer exo-5-(diphenylphosphato)isosorbide-2-endo-acrylate to generate flame retardant poly(styrene). Polymers 2019, 11, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocz, K.; Szolnoki, B.; Marosi, A.; Tábi, T.; Wladyka-Przybylak, M.; Marosi, G. Flax fibre reinforced PLA/TPS biocomposites flame retarded with multifunctional additive system. Polym. Degrad. Stab. 2014, 106, 63–73. [Google Scholar] [CrossRef] [Green Version]
- European standard EN 317:1993; Particleboards and Fibreboards. Determination of Swelling in Thickness after Immersion in Water. British Standards Institution: London, UK, 1993.
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z.; Jin, Y.; Lu, F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym. Degrad. Stab. 2007, 92, 720–726. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel silicon-containing epoxy/PEPA phosphate flame retardant for transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; O’Rear, E.; Yanumet, N. The effect of phosphorus content on the thermal and the burning properties of cotton fabric coated with an ultrathin film of a phosphorus-containing polymer. Polym. Degrad. Stab. 2009, 94, 558–565. [Google Scholar] [CrossRef]
- Bai, Z.; Song, L.; Hu, Y.; Yuen, R. Preparation, flame retardancy, and thermal degradation of unsaturated polyester resin modified with a novel phosphorus containing acrylate. Ind. Eng. Chem. Res. 2013, 52, 12855–12864. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.; Liu, S.; Zhao, J. The synthesis and properties of a reactive flame-retardant unsaturated polyester resin from a phosphorus-containing diacid. Polym. Adv. Technol. 2011, 22, 1768–1777. [Google Scholar] [CrossRef]
- Halpern, J.; Urbanski, R.; Weinstock, A.; Iwig, D.; Mathers, R.; Von Recum, H. A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J. Biomed. Mater. Res.—Part A. 2014, 102, 1467–1477. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, D.; Karak, N. Waterborne sustainable tough hyperbranched aliphatic polyester thermosets. ACS Sustain. Chem. Eng. 2015, 3, 2458–2568. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Mikkola, E.; Laperre, J.; Gensous, F.; Fardell, P.; Le Tallec, Y.; Baiocchi, C.; Paul, K.; Simonson, M.; Deleu, C.; et al. Smoke gas analysis by Fourier transform infrared spectroscopy—Summary of the SAFIR project results. Fire Mater. 2000, 24, 101–112. [Google Scholar] [CrossRef]
- Madejová, J.; Janek, M.; Komadel, P.; Herbert, H.-J.; Moog, H. FTIR analyses of water in MX-80 bentonite compacted from high salinary salt solution systems. Appl. Clay Sci. 2002, 20, 255–271. [Google Scholar] [CrossRef]
- Li, B.; Gonzalez, R. The measurement of small amounts of coke by a sensitive TGA/FTIR technique. Catal. Letters. 1998, 54, 5–8. [Google Scholar] [CrossRef]
- Kauffman, K.; Culp, J.; Goodman, A.; Matranga, C. FT-IR study of CO2 adsorption in a dynamic copper(II) benzoate-pyrazine host with CO2-CO2 interactions in the adsorbed state. J. Phys. Chem. C 2011, 115, 1857–1866. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Blanco-Bonilla, F.; Luna, D.; Bautista, F. Production of acrolein from glycerol in liquid phase on heterogeneous catalysts. Chem. Eng. J. 2015, 282, 179–186. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and cone calorimetry to understand thermal degradation and flame retardancy mechanism of polycarbonate filled with solid bisphenol A bis(diphenyl phosphate) and montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Kök, M.; Varfolomeev, M.; Nurgaliev, D. Crude oil characterization using TGA-DTA, TGA-FTIR and TGA-MS techniques. J. Pet. Sci. Eng. 2017, 154, 537–542. [Google Scholar] [CrossRef]
- Hardy, A.; Van Werde, K.; Vanhoyland, G.; Van Bael, M.; Mullens, J.; Van Poucke, L. Study of the decomposition of an aqueous metal-chelate gel precursor for (Bi, La)4Ti3O12 by means of TGA-FTIR, TGA-MS and HT-DRIFT. Thermochim. Acta. 2003, 397, 143–153. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Liu, T.; Ma, T.; Li, L.; Wang, Q.; Guo, C. Study on degradation of phosphorus and nitrogen composite UV-cured flame retardant coating on wood surface. Prog. Org. Coat. 2018, 124, 240–248. [Google Scholar] [CrossRef]
- Samoudi, B.; Bendaou, O.; Hanafi, I.; Asselman, A.; Haboubi, K. FTIR and Raman Spectroscopy Study of Soot Deposits Produced in the Infrared Multiphoton Dissociation of Vinyl Bromide. J. Spectrosc. 2022, 2022, 11. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A. On the performance and mechanism of brominated and halogen free flame retardants in formulations of glass fibre reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2014, 104, 71–86. [Google Scholar] [CrossRef]
- Pagot, G.; Bertasi, F.; Vezzù, K.; Nawn, G.; Pace, G.; Nale, A.; Di Noto, V. Correlation between Properties and Conductivity Mechanism in Poly(vinyl alcohol)-based Lithium Solid Electrolytes. Solid State Ionics. 2018, 320, 177–185. [Google Scholar] [CrossRef]
- Costa, N.; Pereira, J.; Martins, J.; Ferra, J.; Cruz, P.; Magalhães, F.; Mendes, A.; Carvalho, L. Alternative to latent catalysts for curing UF resins used in the production of low formaldehyde emission wood-based panels. Int. J. Adhes. Adhes. 2012, 33, 56–60. [Google Scholar] [CrossRef]
- Ly, B.; Dyer, E.; Feig, J.; Chien, A.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, A.; Hadden, R.; Bisby, L. A Review of Factors Affecting the Burning Behaviour of Wood for Application to Tall Timber Construction. Fire Technol. 2019, 55, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Grexa, O.; Lübke, H. Flammability parameters of wood tested on a cone calorimeter. Polym. Degrad. Stab. 2001, 74, 427–432. [Google Scholar] [CrossRef]
- Dunky, M. Urea–formaldehyde (UF) adhesive resins for wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, R. Advantages of flame retardants based on nitrogen compounds. Polym. Degrad. Stab. 1996, 54, 205–215. [Google Scholar] [CrossRef]
- Vu, V.; Cloutier, A.; Bissonnette, B.; Blanchet, P.; Dagenais, C. Steatite powder additives in wood-cement drywall particleboards. Materials 2020, 13, 4813. [Google Scholar] [CrossRef]
- Alexiou, P.; Gardner, W. Effect of thickness and board composition on the early fire hazard properties of particleboard. Archit. Sci. Rev. 1986, 29, 77–81. [Google Scholar] [CrossRef]
- Zagozdzon, I.; Parcheta, P.; Datta, J. Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors. Materials 2021, 14, 2699. [Google Scholar] [CrossRef] [PubMed]
- Puyadena, M.; Etxeberria, I.; Martin, L.; Mugica, A.; Agirre, A.; Cobos, M.; Gonzalez, A.; Barrio, A.; Irusta, L. Polyurethane/acrylic hybrid dispersions containing phosphorus reactive flame retardants as transparent coatings for wood. Prog. Org. Coat. 2022, 170, 107005. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Chen, L.; Wang, W.; Zhao, B.; Wang, Y. A main-chain phosphorus-containing poly(trimethylene terephthalate) copolyester: Synthesis, characterization, and flame retardance. Polym. Adv. Technol. 2012, 23, 1276–1282. [Google Scholar] [CrossRef]
- Spontón, M.; Ronda, J.; Galià, M.; Cádiz, V. Cone calorimetry studies of benzoxazine-epoxy systems flame retarded by chemically bonded phosphorus or silicon. Polym. Degrad. Stab. 2009, 94, 102–106. [Google Scholar] [CrossRef]




















| Polymer | Flame Retardant Introduction Mechanism | Bio-Compound | Source |
|---|---|---|---|
| PLA | Phytic acid incorporation by pad-dry-cure technique | Phytic acid and PLA | [15] |
| PLA | Lignin phosphorylation and conversion to ammonium phosphate | Lignin and PLA | [16] |
| PHFC | CEPPA esterification with HDO and FDCA | FDCA | [17] |
| PBT | Epoxidized linseed oil and downstream corn oil phosphorylation | Linseed oil and downstream corn oil | [18] |
| PU-foam | Epoxidized soybean oil phosphorylation | Soybean oil | [19] |
| PU-foam | Polyol based on phenylphosphonic acid and propylene oxide | Limonene | [20] |
| Epoxy resin | Vanillin coupling with diethyl phosphite | Vanillin | [21] |
| Epoxy resin | Vanillin and guaiacol coupling with DOPO | Vanillin and guaiacol | [22] |
| Epoxy resin | Epoxy monomer modification with phloroglucinol | Phloroglucinol | [23] |
| PS | Isosorbide reaction with diphenylchlorophosphate and vinyl group incorporation with acryloyl chloride | Isosorbide | [24] |
| Board | Wood Particles [g] | UF-Resin [g] | Additive/Weight [g] | Thickness [mm] | Average Density [kg·m−3] |
|---|---|---|---|---|---|
| A | 304.4 | 72.74 | - | 10.9 ± 0.07 | 658 ± 50 |
| B | 250.7 | 72.74 | APP/47.28 | 10.8 ± 0.03 | 643 ± 31 |
| C | 250.7 | 72.74 | PE1/47.28 | 11.7 ± 0.12 | 532 ± 63 |
| D | 250.7 | 72.74 | PE2/47.28 | 11.5 ± 0.14 | 566 ± 35 |
| E | 197.0 | 72.74 | PE2/94.56 | 11.0 ± 0.13 | 617 ± 80 |
| F * | 197.0 | 72.74 | PE2/94.56 | 10.4 ± 0.11 | 626 ± 28 |
| Sample | Weight Loss [wt%] | Residue at 800 °C [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| 50–300 °C | 300–600 °C | 600–800 °C | ||||||
| N2 | Air | N2 | Air | N2 | Air | N2 | Air | |
| PG1 | 64.1 | 63.7 | 12.8 | 9.4 | 1.8 | 18.5 | 18.8 | 6.2 |
| PG2 | 48.7 | 47.8 | 18.3 | 18.0 | 4.6 | 27.2 | 24.7 | 5.4 |
| PE1 | 51.9 | 51.0 | 13.4 | 18.6 | 2.8 | 18.8 | 30.7 | 10.3 |
| PE2 | 51.5 | 52.0 | 15.2 | 15.9 | 3.2 | 29.9 | 29.5 | 1.2 |
| Sample | P [%] | tig [s] | PHRR [kW·m−2] | tpeak [s] | THR [MJ·m−2] | MAHRE [kW·m−2] | Residue [%] | TSP [m2] | SEA [m2·kg−1] |
|---|---|---|---|---|---|---|---|---|---|
| A | 0 | 49.0 | 228.2 | 579.3 | 92.6 | 113.7 | 16.6 | 1.9 | 11.8 |
| B | 3.95 | 31.3 | 114.8 | 42.0 | 23.1 | 43.3 | 32.9 | 10.8 | 233.3 |
| C | 0.55 | 22.5 | 165.0 | 632.0 | 70.7 | 83.8 | 20.2 | 1.1 | 16.3 |
| D | 0.92 | 28.0 | 142.7 | 653.0 | 65.2 | 74.6 | 23.3 | 0.9 | 12.3 |
| E | 1.87 | 30.0 | 134.2 | 41.0 | 31.1 | 61.2 | 27.5 | 7.5 | 141.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, I.; Butron, A.; Puyadena, M.; González, A.; Irusta, L.; Barrio, A. Bio-Based Phosphate-Containing Polyester for Improvement of Fire Reaction in Wooden Particleboard. Polymers 2023, 15, 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051093
Svensson I, Butron A, Puyadena M, González A, Irusta L, Barrio A. Bio-Based Phosphate-Containing Polyester for Improvement of Fire Reaction in Wooden Particleboard. Polymers. 2023; 15(5):1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051093
Chicago/Turabian StyleSvensson, Ingemar, Amaia Butron, Maddalen Puyadena, Alba González, Lourdes Irusta, and Aitor Barrio. 2023. "Bio-Based Phosphate-Containing Polyester for Improvement of Fire Reaction in Wooden Particleboard" Polymers 15, no. 5: 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051093