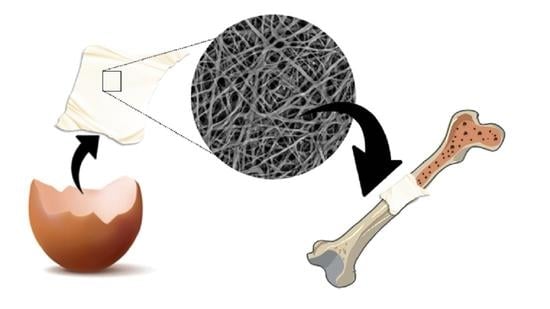
Eggshell Membrane as a Biomaterial for Bone Regeneration
Abstract
:1. Introduction
2. Criteria for the Development of Bone Scaffolds
3. Membrane Extraction
4. Eggshell Membrane Structure and Composition



5. Biological, Physical, and Mechanical Properties of the Eggshell Membrane
5.1. Biological Properties
5.2. Physical Properties
| Property | Extraction | State | Method | Value | Ref. |
|---|---|---|---|---|---|
| Thickness | Manual | Humid | Micrometer | ~0.096 mm | [56] |
| Manual | Not specified | Micrometer | ~0.080 mm | [138] | |
| Shell dissolution (acetic acid) | ~0.124 mm | ||||
| Shell dissolution (EDTA) | ~0.122 mm | ||||
| Manual removal | Dry | Confocal scanning laser microscopy | ~50–70 μm (outer membrane) | [94] | |
| ~15–26 μm (inner membrane) | |||||
| ~3.6 μm (limiting membrane) | |||||
| Porosity | Manual removal | Dry | SEM | 56.54% | [56] |
| Humid (ethanol) | Liquid displacement method | 9.95% | [58] | ||
| Humid (water) | AFM | 52.06% | [2] | ||
| Contact angle | Manual removal | Dry | Contact angle meter. 2 μL PBS microdroplet | Between 40–50° | [56] |
| Manual removal | Not described | Contact angle meter. 10 μL water microdroplet | ~78° | [58] | |
| Shell dissolution (HCl) | Not specified | Drop shape analysis system goniometer. Water microdroplet | Inner membrane: 80.5° (3 s) 46.3° (2 min) Outer membrane: 99.8° (3 s), 68.8° (2 min) | [76] | |
| Burst strength | Manual removal | Wet (PBS) | Texture analyzer | ~2 N | [56] |
| Tensile strength | Manual removal | Wet (PBS) | 0.9 MPa | [56] | |
| Manual removal | Not specified | Tensile testing machine | 0.9–3.2 MPa | [138] | |
| Shell dissolution (acetic acid) | Wet | Texture analyzer | 1.3 MPa | ||
| Shell dissolution (EDTA) | Wet | 1.4 MPa | |||
| Manual removal | N.S | Universal testing machine | 1.6 MPa | [58] | |
| Manual removal | N.S | 1.6 MPa | [59] | ||
| Manual removal | N.S | Tensile testing machine | 6.4 MPa | [2] | |
| Manual removal | Wet (water) | 1.4 MPa | |||
| Wet (albumen) | 1.8 MPa | ||||
| Young’s Modulus | Manual removal | Wet | 4.1 MPa | [56] | |
| Shell dissolution (acetic acid) | Wet (PBS) | Texture analyzer | 3.3 MPa | ||
| Shell dissolution (EDTA) | Wet | 3.6 MPa | |||
| Manual removal | N.S | Universal testing machine | ~4.7–5.5 MPa | [59] | |
| Manual removal | Dry | Tensile testing machine | 232 MPa | [2] | |
| Manual removal | Wet (water) | 5.5 MPa | |||
| Wet (albumen) | 5.3 MPa |
5.3. Mechanical Properties
6. Calcium Phosphate Mineralization of the Eggshell Membrane
7. Limitations of the Present Review
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baláž, M. Eggshell Membrane Biomaterial as a Platform for Applications in Materials Science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Piaggio, F.; Hijar, A. Structure–Property Relationships of a Biopolymer Network: The Eggshell Membrane. Acta Biomater. 2010, 6, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- de Villiers, T.J.; Goldstein, S.R. Bone Health 2022: An Update. Climacteric 2022, 25, 1–3. [Google Scholar] [CrossRef]
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in Bone Tissue Regeneration: Stem Cell Therapy, Biofunctionality and Antimicrobial Properties of Novel Materials and Its Evolution. Int J. Mol. Sci 2020, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Tracy, A.A.; Bhatia, S.K.; Ramadurai, K.W. Impact of Biomaterials on Health and Economic Development. In Bio-Based Materials as Applicable; Accessible, and Affordable Healthcare Solutions; Tracy, A.A., Bhatia, S.K., Ramadurai, K.W., Eds.; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–41. ISBN 978-3-319-69326-2. [Google Scholar]
- Zhang, X.; Li, Q.; Wang, Z.; Zhou, W.; Zhang, L.; Liu, Y.; Xu, Z.; Li, Z.; Zhu, C.; Zhang, X. Bone regeneration materials and their application over 20 years: A bibliometric study and systematic review. Front. Bioeng. Biotechnol. 2022, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric Biomaterials for Bone Regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Fouad, D.; Farag, M. Design for Sustainability with Biodegradable Composites. In Design and Manufacturing; IntechOpen: London, UK, 2019; pp. 1–20. ISBN 978-1-78985-866-2. [Google Scholar]
- Wojnarowska, M.; Sołtysik, M.; Guzik, M. Socio-Economic Importance of Biomaterials in the Transition to the Circular Economy Model. In Proceedings of the 20th International Scientific Conference Globalization and its Socio-Economic Consequences 2020, Rajecke Teplice, Slovakia, 21–22 October 2020; Volume 92, p. 05029. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Zhu, Y.; Narayanan, K.B.; Han, S.S.; Park, J.K. Fabrication Strategies and Biomedical Applications of Three-Dimensional Bacterial Cellulose-Based Scaffolds: A Review. Int. J. Biol. Macromol. 2022, 209, 9–30. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Siddique, R.; Huanfei, D.; Shereen, M.A.; Nabi, G.; Bai, Q.; Manan, S.; Xue, M.; Ullah, M.W.; Bowen, H. Perspective Applications and Associated Challenges of Using Nanocellulose in Treating Bone-Related Diseases. Front. Bioeng. Biotechnol. 2021, 9, 616555. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative Study of Three Different Membranes for Guided Bone Regeneration of Rat Cranial Defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62. [Google Scholar] [CrossRef]
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Front. Bioeng. Biotechnol. 2021, 8, 1522. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K.S.; Lee, D.; Kim, D.; Lim, K.T.; Lee, K.-H.; Seonwoo, H.; Kim, J. Eggshell membrane: Review and impact on engineering. Biosyst. Eng. 2016, 151, 446–463. [Google Scholar] [CrossRef]
- Mahdavi, S.; Amirsadeghi, A.; Jafari, A.; Niknezhad, S.V.; Bencherif, S.A. Avian Egg: A Multifaceted Biomaterial for Tissue Engineering. Ind. Eng. Chem. Res. 2021, 60, 17348–17364. [Google Scholar] [CrossRef]
- Mittal, A.; Teotia, M.; Soni, R.K.; Mittal, J. Applications of egg shell and egg shell membrane as adsorbents: A review. J. Mol. Liq. 2016, 223, 376–387. [Google Scholar] [CrossRef]
- Xiao, N.; Huang, X.; He, W.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Zhao, Y.; Tu, Y. A review on recent advances of egg byproducts: Preparation, functional properties, biological activities and food applications. Food Res. Int. 2021, 147, 1110563. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Sah, M.K.; Rath, S.N. Soluble Eggshell Membrane: A Natural Protein to Improve the Properties of Biomaterials Used for Tissue Engineering Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 807–821. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The Material Bone: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A. The Role of Collagen in Bone Apatite Formation in the Presence of Hydroxyapatite Nucleation Inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Fernández-Penas, R.; Romero-Castillo, I.; Verdugo-Escamilla, C.; Choquesillo-Lazarte, D.; D’Urso, A.; Prat, M.; Fernández-Sánchez, J.F. Crystallization, Luminescence and Cytocompatibility of Hexagonal Calcium Doped Terbium Phosphate Hydrate Nanoparticles. Nanomaterials 2021, 11, 322. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the Preparation of Nanocrystalline Apatites and Surface Characterization: Overview of Fundamental and Applied Aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Edit. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Iafisco, M.; Ramirez-Rodriguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gomez-Morales, J.; Manuel Delgado-Lopez, J. The Growth Mechanism of Apatite Nanocrystals Assisted by Citrate: Relevance to Bone Biomineralization. Crystengcomm 2015, 17, 507–511. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Rawal, A.; Schmidt-Rohr, K. Strongly Bound Citrate Stabilizes the Apatite Nanocrystals in Bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Frison, R.; Cervellino, A.; Gómez-Morales, J.; Guagliardi, A.; Masciocchi, N. Crystal Size, Morphology, and Growth Mechanism in Bio-Inspired Apatite Nanocrystals. Adv. Funct. Mater. 2014, 24, 1090–1099. [Google Scholar] [CrossRef]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired Citrate–Apatite Nanocrystals Doped with Divalent Transition Metal Ions. Cryst. Growth Des. 2016, 16, 145–153. [Google Scholar] [CrossRef]
- Elsharkawy, S.; Mata, A. Hierarchical Biomineralization: From Nature’s Designs to Synthetic Materials for Regenerative Medicine and Dentistry. Adv. Healthc. Mater. 2018, 7, 1800178. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical Basis of Bone Strength: Influence of Bone Material, Bone Structure and Muscle Action. J. Musculoskelet. Neuronal. Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Yousaf, S.; Keshel, S.H.; Farzi, G.A.; Momeni-Moghadam, M.; Ahmadi, E.D.; Mozafari, M.; Sefat, F. 61–Scaffolds for intraocular lens. In Handbook of Tissue Engineering Scaffolds; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; Volume 2, pp. 693–709. [Google Scholar] [CrossRef]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Chapter 7–Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: London, UK, 2019; pp. 85–98. ISBN 978-0-12-812258-7. [Google Scholar]
- Codrea, C.I.; Croitoru, A.-M.; Baciu, C.C.; Melinescu, A.; Ficai, D.; Fruth, V.; Ficai, A. Advances in Osteoporotic Bone Tissue Engineering. J. Clin. Med. 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Scaglione, S. Scaffold Microstructure Effects on Functional and Mechanical Performance: Integration of Theoretical and Experimental Approaches for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2016, 68, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Arias, J.I.; Gonzalez, A.; Fernandez, M.S.; Gonzalez, C.; Saez, D.; Arias, J.L. Eggshell Membrane as a Biodegradable Bone Regeneration Inhibitor. J. Tissue Eng. Regen. Med. 2008, 2, 228–235. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jo, S.B.; Kim, H.; Park, S.-M.; Patel, K.D.; Cho, K.J.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Sidney, L.E.; et al. The Eggshell Membrane: A Potential Biomaterial for Corneal Wound Healing. J. Biomater. Appl. 2021, 36, 912–929. [Google Scholar] [CrossRef]
- Kavarthapu, A.; Malaiappan, S. Comparative Evaluation of Demineralized Bone Matrix and Type II Collagen Membrane versus Eggshell Powder as a Graft Material and Membrane in Rat Model. Indian J. Dent. Res. 2019, 30, 877. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, Y.M.; Suh, J.-Y.; Han, J.Y. Beneficial Effect on Rapid Skin Wound Healing through Carboxylic Acid-Treated Chicken Eggshell Membrane. Mater. Sci. Eng. C 2021, 128, 112350. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, H.; Abdolmaleki, A. Thermo-Chemical Modification of a Natural Biomembrane to Induce Mucoadhesion, PH Sensitivity and Anisotropic Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2018, 87, 50–58. [Google Scholar] [CrossRef]
- Makkar, S.K.; Rath, N.C.; Packialakshmi, B.; Zhou, Z.Y.; Huff, G.R.; Donoghue, A.M. Nutritional Supplement of Hatchery Eggshell Membrane Improves Poultry Performance and Provides Resistance against Endotoxin Stress. PLoS ONE 2016, 11, e0159433. [Google Scholar] [CrossRef]
- Pillai, M.M.; Gopinathan, J.; Senthil Kumar, R.; Sathish Kumar, G.; Shanthakumari, S.; Sahanand, K.S.; Bhattacharyya, A.; Selvakumar, R. Tissue engineering of human knee meniscus using functionalized and reinforced silk-polyvinyl alcohol composite three-dimensional scaffolds. J. Biomed. Mater. Res. A 2017, 106, 1722–1731. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Fu, B.; Li, K.; Huang, D.; Zheng, C.; Liu, M.; Yang, D.-P. Eggshell Membrane-Mimicking Multifunctional Nanofiber for in-Situ Skin Wound Healing. Int. J. Biol. Macromol. 2022, 210, 139–151. [Google Scholar] [CrossRef]
- Bello, M.; Abdullah, F.; Mahmood, W.M.A.W.; Malek, N.A.N.N.; Jemon, K.; Siddiquee, S.; Chee, T.Y.; Sathishkumar, P. Electrospun Poly (Ɛ-Caprolactone)-Eggshell Membrane Nanofibrous Mat as a Potential Wound Dressing Material. Biochem. Eng. J. 2022, 187, 108563. [Google Scholar] [CrossRef]
- 64 Golafshan, N.; Gharibi, H.; Kharaziha, M.; Fathi, M. A Facile One-Step Strategy for Development of a Double Network Fibrous Scaffold for Nerve Tissue Engineering. Biofabrication 2017, 9, 025008. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, I.A.; Serban, A.P.; Nicoara, A.I.; Trusca, R.; Ene, V.L.; Iordache, F. Biomimetic Composite Scaffold Based on Naturally Derived Biomaterials. Polymers 2020, 12, 1161. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, S.; Tang, Y.; Mo, B.; Liao, H. New Zonal Structure and Transition of the Membrane to Mammillae in the Eggshell of Chicken Gallus Domesticus. J. Struct. Biol. 2018, 203, 162–169. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous Calcium Carbonate Controls Avian Eggshell Mineralization: A New Paradigm for Understanding Rapid Eggshell Calcification. J. Struct. Biol. 2015, 190, 291–303. [Google Scholar] [CrossRef]
- Deickert, J.W.; Dieckert, M.C.; Creger, C.R. Calcium Reserve Assembly: A Basic Structural Unit of the Calcium Reserve System of the Hen Egg Shell. Poult. Sci. 1989, 68, 1569–1584. [Google Scholar] [CrossRef]
- Kim, D.; Gwon, Y.; Park, S.; Kim, W.; Yun, K.; Kim, J. Eggshell Membrane as a Bioactive Agent in Polymeric Nanotopographic Scaffolds for Enhanced Bone Regeneration. Biotechnol. Bioeng. 2021, 118, 1862–1875. [Google Scholar] [CrossRef]
- Hsieh, S.; Chou, H.-H.; Hsieh, C.-W.; Wu, D.-C.; Kuo, C.-H.; Lin, F.-H. Hydrogen Peroxide Treatment of Eggshell Membrane to Control Porosity. Food Chem. 2013, 141, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Yu-Hong, Z.; Yu-Jie, C. Characterization of Collagen from Eggshell Membrane. Biotechnology 2009, 8, 254–258. [Google Scholar] [CrossRef]
- Tsai, W.; Yang, J.; Lai, C.; Cheng, Y.; Lin, C.; Yeh, C. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, L.; Chi, Y. Extracting Hyaluronic Acid From Eggshell Membrane With Enzyme. Food Res. Dev. 2008, 29, 40–43. [Google Scholar]
- Ray, P.G.; Roy, S. Eggshell Membrane: A Natural Substrate for Immobilization and Detection of DNA. Mater. Sci. Eng. C 2016, 59, 404–410. [Google Scholar] [CrossRef]
- Baláž, M.; Zorkovská, A.; Fabián, M.; Girman, V.; Briančin, J. Eggshell Biomaterial: Characterization of Nanophase and Polymorphs after Mechanical Activation. Adv. Powder Technol 2015, 26, 1597–1608. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Wen, W.; Lu, L.; Luo, B.; Zhou, C. Biomimetic Mineralisation of Eggshell Membrane Featuring Natural Nanofiber Network Structure for Improving Its Osteogenic Activity. Colloids Surf. B Biointerfaces 2019, 179, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Armitage, O.E.; Strange, D.G.T.; Oyen, M.L. Biomimetic Calcium Carbonate–Gelatin Composites as a Model System for Eggshell Mineralization. J. Mater. Res. 2012, 27, 3157–3164. [Google Scholar] [CrossRef]
- Torres-Mansilla, A.C.; Delgado-Mejía, E. Influence of Separation Techniques with Acid Solutions on the Composition of Eggshell Membrane. Int. J. Poult. Sci. 2017, 16, 451–456. [Google Scholar] [CrossRef]
- Rose-Martel, M.; Smiley, S.; Hincke, M.T. Novel Identification of Matrix Proteins Involved in Calcitic Biomineralization. J. Proteomics 2015, 116, 81–96. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Diep, T.; Hudson, H.-A.; Hincke, M.T. High Value Applications and Current Commercial Market for Eggshell Membranes and Derived Bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef]
- Hussain, A.; Dev, S.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Separation of Egghsell and Membrane. In Proceedings of the XVIIth World Congress of The International Commission of Agricultural and Biosystems Engineering (CIGR), Québec, QC, Canada, 13–17 June 2010. [Google Scholar]
- Chi, Y.; Liu, R.; Lin, M.; Chi, Y. A Novel Process to Separate the Eggshell Membranes and Eggshells via Flash Evaporation. Food Sci. Tech. 2022, 42. [Google Scholar] [CrossRef]
- Yoo, S.; Hsieh, J.S.; Zou, P.; Kokoszka, J. Utilization of Calcium Carbonate Particles from Eggshell Waste as Coating Pigments for Ink-Jet Printing Paper. Bioresour Technol. 2009, 100, 6416–6421. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Rovinaru, C. Separation Methods of the Eggshell Membranes from Eggshell. Proceedings 2019, 29, 9122. [Google Scholar] [CrossRef]
- Snyder, T. Eggshell Membrane Separation Process. U.S. Patent 9,370, 778 B2, 21 June 2016. [Google Scholar]
- Vlad, V. Eggshell Membrane Separation Method. U.S. Patent 7,954,733 B2, 7 June 2011. [Google Scholar]
- New, L. Eggshell Membrane Separation Process. U.S. Patent 8,448,884 B2, 28 May 2013. [Google Scholar]
- Thoroski, J. Eggshell Processing Methods and Apparatus. U.S. Patent 6,649,203 B1, 18 November 2003. [Google Scholar]
- Utgård, B.; Amundsen, S.; Suso, H.-P. Method of Processing Eggshell Residues. WIPO (PCT) patent WO2015/058790, 30 April 2015. [Google Scholar]
- MacNeil, J.H. Method and Apparatus for Separating a Protein Membrane and Shell Material in Waste Egg Shells. U.S. Patent 6,176,376 B1, 23 January 2001. [Google Scholar]
- Ahmed, T.A.E.; Suso, H.-P.; Hincke, M.T. In-Depth Comparative Analysis of the Chicken Eggshell Membrane Proteome. J. Proteom. 2017, 155, 49–62. [Google Scholar] [CrossRef]
- Yang, Q.-S.; Li, S.-W.; Zhu, J.-Q.; Li, X. An Investigation on the Viscoelastic Behavior of Eggshell Membrane by Nanoindentation Technology. Int. J. Appl. Mech. 2019, 11, 1950078. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The Eggshell: Structure, Composition and Mineralization. Front. Biosci 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Liong, J.W.W.; Frank, J.F.; Bailey, S. Visualization of Eggshell Membranes and Their Interaction with Salmonella Enteritidis Using Confocal Scanning Laser Microscopy. J. Food Prot. 1997, 60, 1022–1028. [Google Scholar] [CrossRef]
- Hincke, M.T.; Gautron, J.; Panheleux, M.; Garcia-Ruiz, J.; McKee, M.D.; Nys, Y. Identification and Localization of Lysozyme as a Component of Eggshell Membranes and Eggshell Matrix. Matrix Biol. 2000, 19, 443–453. [Google Scholar] [CrossRef]
- Li, N.; Niu, L.; Qi, Y.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.; Pashley, D.H.; Tay, F.R. Subtleties of Biomineralisation Revealed by Manipulation of the Eggshell Membrane. Biomaterials 2011, 32, 8743–8752. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Arias, J.I.; Fernandez, M.S. Avian Eggshell as a Template for Biomimetic Synthesis of New Materials. In Handbook of Biomineralization; Buerlein, E., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; pp. 109–117. ISBN 978-3-527-61944-3. [Google Scholar]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian Eggshell Mineralization: Biochemical and Functional Characterization of Matrix Proteins. Comptes. Rendus. Palevol. 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Bellairs, R.; Boyde, A. Scanning Electron Microscopy of the Shell Membranes of the Hen’s Egg. Z. Für Zellforsch. Und Mikrosk. Anat. 1969, 96, 237–249. [Google Scholar] [CrossRef]
- Rao, A.; Fernández, M.S.; Cölfen, H.; Arias, J.L. Distinct Effects of Avian Egg Derived Anionic Proteoglycans on the Early Stages of Calcium Carbonate Mineralization. Cryst. Growth Des. 2015, 15, 2052–2056. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like Agglomerates of Hydroxyapatite Crystals Formed on an Egg-Shell Membrane. Colloids Surf. B: Biointerfaces. 2011, 82, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.S.; Passalacqua, K.; Arias, J.I.; Arias, J.L. Partial Biomimetic Reconstitution of Avian Eggshell Formation. J. Struct. Biol. 2004, 148, 1–10. [Google Scholar] [CrossRef]
- Fernandez, M.S.; Moya, A.; Lopez, L.; Arias, J.L. Secretion Pattern, Ultrastructural Localization and Function of Extracellular Matrix Molecules Involved in Eggshell Formation. Matrix Biol. 2001, 19, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ikawa, N.I.; Ozimek, L. Chemical Composition of Chicken Eggshell and Shell Membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Kodali, V.K.; Gannon, S.A.; Paramasivam, S.; Raje, S.; Polenova, T.; Thorpe, C. A Novel Disulfide-Rich Protein Motif from Avian Eggshell Membranes. PLoS ONE 2011, 6, e18187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Suso, H.-P.; Maqbool, A.; Hincke, M.T. Processed Eggshell Membrane Powder: Bioinspiration for an Innovative Wound Healing Product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.A.; Dennis, J.E.; Wu, T.M.; Arias, J.L.; Fernandez, M.S.; Rodriguez, J.P.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. The Avian Eggshell Extracellular Matrix as a Model for Biomineralization. Connect. Tissue Res. 1996, 35, 325–329. [Google Scholar] [CrossRef]
- Wong, M.; Hendrix, M.J.; von der Mark, K.; Little, C.; Stern, R. Collagen in the Egg Shell Membranes of the Hen. Dev. Biol. 1984, 104, 28–36. [Google Scholar] [CrossRef]
- Kaweewong, K.; Garnjanagoonchorn, W.; Jirapakkul, W.; Roytrakul, S. Solubilization and Identification of Hen Eggshell Membrane Proteins During Different Times of Chicken Embryo Development Using the Proteomic Approach. Protein J. 2013, 32, 297–308. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernandez, M.S.; Dennis, J.E.; Caplan, A.I. Collagens of the Chicken Eggshell Membranes. Connect. Tissue Res. 1991, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.C.; Liyanage, R.; Makkar, S.K.; Lay, J.O. Protein Profiles of Hatchery Egg Shell Membrane. Proteome. Sci. 2017, 15, 4. [Google Scholar] [CrossRef]
- Bayraktar, O.; Galanakis, C.M.; Aldawoud, T.M.S.; Ibrahim, S.A.; Köse, M.D.; Uslu, M.E. Utilization of Eggshell Membrane and Olive Leaf Extract for the Preparation of Functional Materials. Foods 2021, 10, 806. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.-T.; Lim, T.-T.; Yan, X. Facile Room-Temperature Synthesis of Carboxylated Graphene Oxide-Copper Sulfide Nanocomposite with High Photodegradation and Disinfection Activities under Solar Light Irradiation. Sci Rep. 2015, 5, 16369. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Kaiden, K.; Matsui, T.; Tanaka, S. A Study of the Amide III Band by FT-IR Spectrometry of the Secondary Structure of Albumin, Myoglobin, and γ-Globulin. Appl. Spectrosc. 1987, 41, 180–184. [Google Scholar] [CrossRef]
- Biswas, N.; Waring, A.J.; Walther, F.J.; Dluhy, R.A. Structure and Conformation of the Disulfide Bond in Dimeric Lung Surfactant Peptides SP-B1–25 and SP-B8–25. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Notingher, I.; Green, C.; Dyer, C.; Perkins, E.; Hopkins, N.; Lindsay, C.; Hench, L.L. Discrimination between Ricin and Sulphur Mustard Toxicity in Vitro Using Raman Spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Shepherd, N.; Crow, P.; Barr, H. Near-Infrared Raman Spectroscopy for the Classification of Epithelial Pre-Cancers and Cancers. J. Raman Spectrosc. 2002, 33, 564–573. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman Lines of Phenylalanine Analyzed by a Multiconformational Approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near Infrared Raman Spectra of Human Brain Lipids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1529–1535. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman Spectroscopy of Proteins: A Review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Cheng, W.-T.; Liu, M.-T.; Liu, H.-N.; Lin, S.-Y. Micro-Raman Spectroscopy Used to Identify and Grade Human Skin Pilomatrixoma. Microsc. Res. Tech. 2005, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the Mechanical Properties of Biomaterials on Degradability, Cell Behaviors and Signaling Pathways: Current Progress and Challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Rønning, S.B.; Berg, R.S.; Høst, V.; Veiseth-Kent, E.; Wilhelmsen, C.R.; Haugen, E.; Suso, H.-P.; Barham, P.; Schmidt, R.; Pedersen, M.E. Processed Eggshell Membrane Powder Is a Promising Biomaterial for Use in Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 8130. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.T.; Rønning, S.B.; Ahmed, T.A.E.; Brathagen, K.; Høst, V.; Hincke, M.T.; Suso, H.-P.; Pedersen, M.E. Processed Eggshell Membrane Powder Regulates Cellular Functions and Increase MMP-Activity Important in Early Wound Healing Processes. PLoS ONE 2018, 13, e0201975. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Ahn, J.; Lim, W.; Son, D.J.; Lee, E.; Lim, T.-G. Anti-Skin Aging Activity of Eggshell Membrane Administration and Its Underlying Mechanism. Mol. Cell Toxicol. 2022, 19, 165–176. [Google Scholar] [CrossRef]
- Essary, E.O.; Sheldon, B.W.; Crews, S.L. Relationship Between Shell and Shell Membrane Strength and Other Egg Shell Characteristics. Poult. Sci. 1977, 56, 1882–1888. [Google Scholar] [CrossRef]
- Peterson, S.H.; Ackerman, J.T.; Herzog, M.P.; Toney, M.S.; Cooney, B.; Hartman, C.A. Avian Eggshell Thickness in Relation to Egg Morphometrics, Embryonic Development, and Mercury Contamination. Ecol. Evol. 2020, 10, 8715–8740. [Google Scholar] [CrossRef]
- Castilla, A.M.; Van Dongen, S.; Herrel, A.; Francesch, A.; Martínez de Aragón, J.; Malone, J.; José Negro, J. Increase in Membrane Thickness during Development Compensates for Eggshell Thinning Due to Calcium Uptake by the Embryo in Falcons. Naturwissenschaften 2010, 97, 143–151. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Strnková, J.; Nedomová, Š.; Kumbár, V.; Trnka, J. Tensile Strength of the Eggshell Membranes. Acta Univ. Agric. Silvic. Mendelianae Brun. 2016, 64, 159–164. [Google Scholar] [CrossRef]
- Corominas-Murtra, B.; Petridou, N.I. Viscoelastic Networks: Forming Cells and Tissues. Front. Phys. 2021, 9, 314. [Google Scholar] [CrossRef]
- Hussain, S.H.; Limthongkul, B.; Humphreys, T.R. The Biomechanical Properties of the Skin. Dermatol. Surg. 2013, 39, 193–203. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer Publisher: New York, NY, USA, 2013; pp. 321–391. ISBN 978-1-4757-2257-4. [Google Scholar]
- Kemps, B.J.; Govaerts, T.; De Ketelaere, B.; Mertens, K.; Bamelis, F.R.; Bain, M.M.; Decuypere, E.M.; De Baerdemaeker, J.G. The Influence of Line and Laying Period on the Relationship between Different Eggshell and Membrane Strength Parameters. Poult. Sci. 2006, 85, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Risteli, L.; Koivula, M.-K.; Risteli, J. Procollagen Assays in Cancer. Adv. Clin. Chem. 2014, 66, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Hassenkam, T.; Grant, C.A.; Magnusson, S.P. Tensile Properties of Human Collagen Fibrils and Fascicles Are Insensitive to Environmental Salts. Biophys. J. 2010, 99, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, H. Chapter 5–Egg Components in Food Systems. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: San Diego, United States, 2013; pp. 215–241. ISBN 978-0-12-242352-9. [Google Scholar]
- Strasser, S.; Zink, A.; Janko, M.; Heckl, W.M.; Thalhammer, S. Structural Investigations on Native Collagen Type I Fibrils Using AFM. Biochem. Biophys. Res. Commun. 2007, 354, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Vinckier, A.; Semenza, G. Measuring Elasticity of Biological Materials by Atomic Force Microscopy. FEBS Lett. 1998, 430, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kelc, R.; Naranda, J.; Matevz, K.; Vogrin, M. The Physiology of Sports Injuries and Repair Processes. In Current Issues in Sports and Exercise Medicine; IntechOpen: London, UK, 2013; pp. 43–86. ISBN 978-953-51-1031-6. [Google Scholar]
- Xu, Z.; Neoh, K.G.; Kishen, A. A Biomimetic Strategy to Form Calcium Phosphate Crystals on Type I Collagen Substrate. Mater. Sci. Eng. C 2010, 30, 822–826. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Ahmed, T.A.E.; Wu, L.; Diep, T.; Hincke, M.T. A Novel Eco-Friendly Green Approach to Produce Particalized Eggshell Membrane (PEM) for Skin Health Applications. Biomater. Sci. 2020, 8, 5346–5361. [Google Scholar] [CrossRef]
- Ma, H.; Yang, C.; Ma, Z.; Wei, X.; Younis, M.R.; Wang, H.; Li, W.; Wang, Z.; Wang, W.; Luo, Y.; et al. Multiscale Hierarchical Architecture-Based Bioactive Scaffolds for Versatile Tissue Engineering. Adv. Healthc. Mater. 2022, 11, 2102837. [Google Scholar] [CrossRef] [PubMed]






| Acid | Concentration | Soaking Time | Temperature | Ref. |
|---|---|---|---|---|
| Acetic Acid | 70% w/w | Two days | N.S 1 | [72] |
| 2 % w/w | 30 min | N.S | [74] | |
| 1% | 10 min 2 | N.S | [55] | |
| 0.5 M | 44 h | RT 3 | [56] | |
| HCl | 1 M | N. S | N. S | [65] |
| 0.03 M | 10 min | ~100 °C | [75] | |
| 1 M | One hour | 25 °C | [76] | |
| EDTA | 5% w/w | One day | N.S | [77] |
| n-butyl acetate | 5% w/w | 30 min | RT | [74] |
| Method | Pros | Cons |
|---|---|---|
| Manual peeling |
|
|
| Chemical dissolution |
| |
| Microwave detachment |
|
|
| Flash evaporation [82]. |
|
|
| Mechanical appliances |
|
|
| Enzymatic method |
|
|
| Band Position (cm−1) | Intensity | Vibration Description |
|---|---|---|
| 1076 | w | (Attributed to polysaccharides) |
| 1239 | w | Amine C-N stretching (Amide III) |
| 1443 | w | CH2 scissoring (attributed to sulfates) |
| 1513 | s | C-N stretching/NH bending (Amide II) |
| 1633 | vs | Amide C=O stretching (Amide I) |
| 2426 | vw | Sulfhydryl group (-SH) 1 |
| 2957 | m | C-H stretching (attributed to lipids) |
| 3272 | S | O-H and N-H stretching (Amide A) |
| Band Position (cm−1) | Intensity | Vibration Description |
|---|---|---|
| 485 | vs | ν(S–S) stretching vibration |
| 650 | m | Tyrosine and phenylalanine C-C twisting mode |
| 750 | m | Symmetric breathing of tryptophan |
| 1010 | m | Phenyl ring angular bending vibrations, related to phenylalanine |
| 1136 | s | Lipids |
| 1240 | s | Amide III |
| 1340 | s | CH deformation (proteins and carbohydrates) |
| 1460 | s | CH2 wagging, CH2/CH3 deformation for lipids and collagen |
| 1668 | vs | Amide I |
| 2440 | w | OH stretching vibrations |
| 2927 | s | CH2 asymmetric stretch |
| Cellular Tests | |||||
| Test | Cell Tested | Biomaterial | Method | Time Span | Ref. |
| Cytotoxicity, cell attachment, and cell proliferation. | Corneal mesenchymal stromal cells (C-MSC) | Untreated natural ESM for corneal wound healing | Cell culture | 1, 3, and 7 days | [56] |
| Human dermal fibroblasts (hDF) (GIBCO and C0135C). | Untreated, natural ESM, and ESM treated with acetic and citric acid | MTT assay | 1, 2, and 3 days | [58] | |
| Osteosarcoma fibroblast-like MG-63 | Modified ESM with citric acid for drug delivery systems and tissue engineering | MTT assay | 1 and 2 days | [59] | |
| Animal Testing | |||||
| Test | Animal Tested | Biomaterial | Method | Time Span | Ref. |
| In vivo skinwound healing | Male Sprague-Dawley rats (7-weeks-old, weighing 200–230 g) | Untreated, natural ESM, and ESM treated with acetic and citric acid | 10 mm skin injuries. Histological and immunohistochemical evaluation | 0, 3, 7, and 10 days | [58] |
| Bone regeneration membrane | Wistar rats | Hydrolyzed ESM treated with pepsin and acetic acid as a membrane for guided bone regeneration | 6 mm calvaria defects. Radiographical and histological examination | 60 days | [23] |
| Subcutaneous implantation | Sprague-Dawley white rats | Acid removed and sterilized ESM as an anti-bone bridging membrane | Paravertebral implantation | 1, 2, 4, 6, and 16 weeks | [55] |
| Anti-bone bridging implantation | New Zealand white rabbits | Acid removed and sterilized ESM as an anti-bone bridging membrane | Rabbit ostectomy and implantation | Between 8 and 16 weeks | [55] |
| Guided bone regeneration membrane | Wistar rats | Untreated, natural ESM for guided bone regeneration. | Periodontal defect performed filled with eggshell powder and covered with ESM. Histological observations | 45 days | [57] |
| Membrane Preparation | Membrane Pre-Treatment | Mineralization Procedure | Time Span | Mineral Detected | Method to Identify the Mineral | Mineral Characteristics | Mechanical Tests | Biological Tests | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Shell dissolution (HCl) | Pepsin, SMTP (also without SMTP), Ca(OH)2 | HEPES solution incubation | 1–4 weeks | Without SMTP: amorphous calcium phosphate. SMTP: hydroxyapatite | XRD, FTIR | Without SMTP: plate-like crystals. SMTP: needle-like crystals. | Microhardness | None. | [149] |
| Manual extraction | None | Membrane placed as a barrier between K2HPO4 and calcium acetate. | 3–12 days | Mixture of calcium hydrogen phosphate and hydroxyapatite, crystalline hydroxyapatite | XRD, TEM | Flower-like crystals. | None | None | [101] |
| Shell dissolution (HCl) | None | 1.5 SBF incubation | 1–7 days | Hydroxyapatite | XRD, FTIR | Globular | None | Cell culture on MC3T3-E1 mouse- pre-osteoblasts. ALP assay, osteogenesis-related-gene protein expression assay. Western blot. | [76] |
| Manual extraction | 3-mercaptopropionic acid, acetic acid, STPP | Incubation in CaCl2, K2HPO4, HEPES, and polyacrylic acid | 14 or 28 days. | Calcium phosphate, apatite, silica nanoparticles. | TEM, FTIR | Nanoplatelets | Nanoindentation. | None. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Mansilla, A.; Hincke, M.; Voltes, A.; López-Ruiz, E.; Baldión, P.A.; Marchal, J.A.; Álvarez-Lloret, P.; Gómez-Morales, J. Eggshell Membrane as a Biomaterial for Bone Regeneration. Polymers 2023, 15, 1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15061342
Torres-Mansilla A, Hincke M, Voltes A, López-Ruiz E, Baldión PA, Marchal JA, Álvarez-Lloret P, Gómez-Morales J. Eggshell Membrane as a Biomaterial for Bone Regeneration. Polymers. 2023; 15(6):1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15061342
Chicago/Turabian StyleTorres-Mansilla, Adriana, Maxwell Hincke, Ana Voltes, Elena López-Ruiz, Paula Alejandra Baldión, Juan Antonio Marchal, Pedro Álvarez-Lloret, and Jaime Gómez-Morales. 2023. "Eggshell Membrane as a Biomaterial for Bone Regeneration" Polymers 15, no. 6: 1342. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15061342