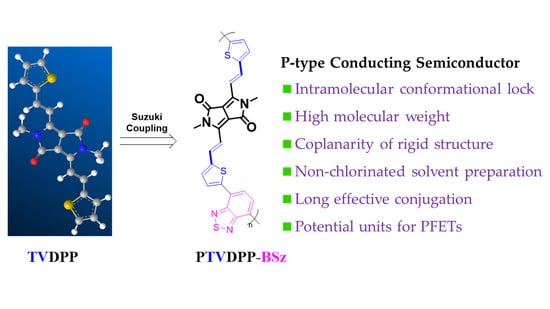
Design of Novel Functional Conductive Structures and Preparation of High-Hole-Mobility Polymer Transistors by Green Synthesis Using Acceptor–Donor–Acceptor Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Procedures for Small-Molecule Intermediates and Monomer
2.2. Synthesis Procedure for Conductive Polymers PTVDPP-BSz
2.3. Characterization Steps and Details of the Intermediates and Polymer
2.4. Device Preparation for Measuring Conductivity of Material
3. Results
3.1. Preparation Route and Molecular Composition Analysis of the Polymer PTVDPP-BSz
3.2. Theoretical Simulation Calculations
3.3. Photochemical Properties
3.4. Electrochemical Properties
3.5. PFET Device Performance
3.6. Microstructure and Morphological Analysis of PTVDPP-BSz Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Hu, W.; Sirringhaus, H.; Müllen, K. Recent Progress in Emerging Organic Semiconductors. Adv. Mater. 2022, 34, 2108701–2108704. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Chen, Y.; Wu, Z.; Chen, Z.; Zhao, Y.; Wang, Y.; Liu, Y. Perylenediimide regioisomers with tunable physicochemical and charge-transport properties. Chem. Commun. 2023, 59, 9876–9879. [Google Scholar] [CrossRef]
- Holliday, S.; Donaghey, J.E.; McCulloch, I. Advances in Charge Carrier Mobilities of Semiconducting Polymers Used in Organic Transistors. Chem. Mater. 2013, 26, 647–663. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Insight into conjugated polymers for organic electrochemical transistors. Trends Chem. 2023, 5, 279–294. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Wang, S.; Guo, Y.; Liu, Y. Insight into High-Performance Conjugated Polymers for Organic Field-Effect Transistors. Chem 2018, 4, 2748–2785. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Voss, G.; Sariciftci, N.S. 25th Anniversary Article: Progress in Chemistry and Applications of Functional Indigos for Organic Electronics. Adv. Mater. 2013, 25, 6783–6800. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, Y.; Wei, D. Two-Dimensional Field-Effect Transistor Sensors: The Road toward Commercialization. Chem. Rev. 2022, 122, 10319–10392. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, Y. Control over the aggregated structure of donor–acceptor conjugated polymer films for high-mobility organic field-effect transistors. Aggregate 2024, 501–525. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Liu, Y. Wearable Electronics Based on Stretchable Organic Semiconductors. Small 2023, 19, 2206309–2206329. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dye. Pigment. 2023, 213, 111136–111147. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Facchetti, A. High-Performance n-Type Polymer Semiconductors: Applications, Recent Development, and Challenges. Chem 2020, 6, 1310–1326. [Google Scholar] [CrossRef]
- Zhu, M.; Guo, Y.; Liu, Y. A thriving decade: Rational design, green synthesis, and cutting-edge applications of isoindigo-based conjugated polymers in organic field-effect transistors. Sci. China Chem. 2022, 65, 1225–1264. [Google Scholar] [CrossRef]
- Ko, E.Y.; Park, G.E.; Lee, D.H.; Um, H.A.; Shin, J.; Cho, M.J.; Choi, D.H. Enhanced Performance of Polymer Solar Cells Comprising Diketopyrrolopyrrole-Based Regular Terpolymer Bearing Two Different π-Extended Donor Units. ACS Appl. Mater. Interfaces 2015, 7, 28303–28310. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th Anniversary Article: Recent Advances in n-Type and Ambipolar Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 5372–5391. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Habibi, A.; Ni, P.; Zhang, Y.; Yassar, A. Tuning the Photophysical Properties of Acceptor–Donor–Acceptor Di-2-(2-oxindolin-3-ylidene) Malononitrile Materials via Extended π–Conjugation: A Joint Experimental and Theoretical Study. Materials 2023, 16, 6410. [Google Scholar] [CrossRef]
- Ding, L.; Yu, Z.-D.; Wang, X.-Y.; Yao, Z.F.; Lu, Y.; Yang, C.-Y.; Wang, J.Y.; Pei, J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123, 7421–7497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, W.; Yu, G. Recent structural evolution of lactam- and imide-functionalized polymers applied in organic field-effect transistors and organic solar cells. Chem. Sci. 2021, 12, 6844–6878. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1711. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2019, 32, 1903882–1903928. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Y.; Hu, W.; Liu, Y. Design and effective synthesis methods for high-performance polymer semiconductors in organic field-effect transistors. Mater. Chem. Front. 2017, 1, 2423–2456. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Mueller, C.J.; Singh, C.R.; Fried, M.; Huettner, S.; Thelakkat, M. High Bulk Electron Mobility Diketopyrrolopyrrole Copolymers with Perfluorothiophene. Adv. Funct. Mater. 2015, 25, 2725–2736. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Singh, S.P.; Soh, M.S.; van Meurs, M.; Tan, J. Annealing-Free High-Mobility Diketopyrrolopyrrole−Quaterthiophene Copolymer for Solution-Processed Organic Thin Film Transistors. J. Am. Chem. Soc. 2011, 133, 2198–2204. [Google Scholar] [CrossRef]
- Li, Y.; Singh, S.P.; Sonar, P. A High Mobility P-Type DPP-Thieno[3,2-b]thiophene Copolymer for Organic Thin-Film Transistors. Adv. Mater. 2010, 22, 4862–4866. [Google Scholar] [CrossRef]
- Hong, J.; Kim, J.; Li, Z.; Cong, C.; Rand, B.P.; Nam, S.Y.; Kim, S.H.; Kim, Y.H. Facile Direct Printing of DPP-Based Polymers for Organic Field-Effect Transistors and Logic Gates. ACS Appl. Electron. Mater. 2023, 5, 4114–4124. [Google Scholar] [CrossRef]
- Kim, H.S.; Huseynova, G.; Noh, Y.-Y.; Hwang, D.-H. Modulation of Majority Charge Carrier from Hole to Electron by Incorporation of Cyano Groups in Diketopyrrolopyrrole-Based Polymers. Macromolecules 2017, 50, 7550–7558. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent Advances in the Development of Semiconducting DPP-Containing Polymers for Transistor Applications. Adv. Mater. 2012, 25, 1859–1880. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, W.; Zhao, Y.; Wang, Y.; Liu, Y. A Hybrid Acceptor-Modulation Strategy: Fluorinated Triple-Acceptor Architecture for Significant Enhancement of Electron Transport in High-Performance Unipolar n-Type Organic Transistors. Adv. Mater. 2022, 35, 2210093–2210104. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Lewis, W.; Amabilino, D.B. Solid state supramolecular structure of diketopyrrolopyrrole chromophores: Correlating stacking geometry with visible light absorption. CrystEngComm 2016, 18, 8933–8943. [Google Scholar] [CrossRef]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type Organic Semiconductors in Organic Electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef]
- Humphreys, J.; Pop, F.; Hume, P.A.; Murphy, A.S.; Lewis, W.; Davies, E.S.; Argent, S.P.; Amabilino, D.B. Solid state structure and properties of phenyl diketopyrrolopyrrole derivatives. CrystEngComm 2021, 23, 1796–1814. [Google Scholar] [CrossRef]
- Zou, X.; Cui, S.; Li, J.; Wei, X.; Zheng, M. Diketopyrrolopyrrole Based Organic Semiconductor Materials for Field-Effect Transistors. Front. Chem. 2021, 9, 671294–671300. [Google Scholar] [CrossRef]
- Li, W.; Lee, T.; Oh, S.J.; Kagan, C.R. Diketopyrrolopyrrole-Based π-Bridged Donor–Acceptor Polymer for Photovoltaic Applications. ACS Appl. Mater. Interfaces 2011, 3, 3874–3883. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, K.; Lai, J.; Zhou, Y.; Wei, X.; Che, Q.; Wei, J.; Wang, L.; Yu, G. Record-High Electron Mobility Exceeding 16 cm2 V−1 s−1 in Bisisoindigo-Based Polymer Semiconductor with a Fully Locked Conjugated Backbone. Adv. Mater. 2023, 35, 2300145–2300155. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, W.; Zhao, Y.; Liu, Y.; Wang, Y. An all-C–H-activation strategy to rapidly synthesize high-mobility well-balanced ambipolar semiconducting polymers. Matter 2022, 5, 1953–1968. [Google Scholar] [CrossRef]
- Ran, Y.; Guo, Y.; Liu, Y. Organostannane-free polycondensation and eco-friendly processing strategy for the design of semiconducting polymers in transistors. Mater. Horiz. 2020, 7, 1955–1970. [Google Scholar] [CrossRef]
- Wang, Y.; Michinobu, T. Benzothiadiazole and its π-extended, heteroannulated derivatives: Useful acceptor building blocks for high-performance donor–acceptor polymers in organic electronics. J. Mater. Chem. C 2016, 4, 6200–6214. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Z.; Ding, L.; Zhang, S.; Chen, Z.; Li, W.; Zhao, Y.; Wang, Y.; Liu, Y. Manipulating Crystal Stacking by Sidechain Engineering for High-Performance N-Type Organic Semiconductors. Adv. Funct. Mater. 2023, 33, 2304316–2304326. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Z.; Huang, H.; Zhang, J.; Yin, Z.; Yu, X.; Zhang, X.s.; Li, C.; Zhang, G.; Huang, M.; et al. High-Performance Ambipolar and n-Type Emissive Semiconductors Based on Perfluorophenyl-Substituted Perylene and Anthracene. Adv. Sci. 2023, 10, 2300530–2300541. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, W.; Wang, X.; Tu, L.; Li, M.; Zhao, Y.; Wang, Y.; Liu, Y. Isomeric Acceptor–Acceptor Polymers: Enabling Electron Transport with Strikingly Different Semiconducting Properties in n-Channel Organic Thin-Film Transistors. Chem. Mater. 2022, 34, 1403–1413. [Google Scholar] [CrossRef]
- Wang, Y.; Kadoya, T.; Wang, L.; Hayakawa, T.; Tokita, M.; Mori, T.; Michinobu, T. Benzobisthiadiazole-based conjugated donor–acceptor polymers for organic thin film transistors: Effects of π-conjugated bridges on ambipolar transport. J. Mater. Chem. C 2015, 3, 1196–1207. [Google Scholar] [CrossRef]
- Luo, X.; Shen, H.; Perera, K.; Tran, D.T.; Boudouris, B.W.; Mei, J. Designing Donor–Acceptor Copolymers for Stable and High-Performance Organic Electrochemical Transistors. ACS Macro Lett. 2021, 10, 1061–1067. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09W, revision A. 02.; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Shen, T.; Zhao, Y.; Liu, Y.; Wang, Y. The marriage of dual-acceptor strategy and C-H activation polymerization: Naphthalene diimide-based n-type polymers with adjustable molar mass and decent performance. Sci. China Chem. 2022, 66, 548–561. [Google Scholar] [CrossRef]
- Jeong, W.; Lee, K.; Jang, J.; Jung, I.H. Development of Benzobisoxazole-Based Novel Conjugated Polymers for Organic Thin-Film Transistors. Polymers 2023, 15, 1156. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.; Tong, M.; Seo, J.H.; Yang, C. Poly(diketopyrrolopyrrole-benzothiadiazole) with Ambipolarity Approaching 100% Equivalency. Adv. Funct. Mater. 2011, 21, 1910–1916. [Google Scholar] [CrossRef]
- Gruber, M.; Jung, S.-H.; Schott, S.; Venkateshvaran, D.; Kronemeijer, A.J.; Andreasen, J.W.; McNeill, C.R.; Wong, W.W.H.; Shahid, M.; Heeney, M.; et al. Enabling high-mobility, ambipolar charge-transport in a DPP-benzotriazole copolymer by side-chain engineering. Chem. Sci. 2015, 6, 6949–6960. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Chen, J.; Huang, J.; Jiang, Y.; Zhang, J.; Shi, L.; Sun, Y.; Wei, Z.; Yu, G.; et al. Bis-Diketopyrrolopyrrole Moiety as a Promising Building Block to Enable Balanced Ambipolar Polymers for Flexible Transistors. Adv. Mater. 2017, 29, 1606162–1606169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, A.R.; Hong, J.; Kim, G.; Lee, J.; Shin, T.J.; Oh, J.H.; Yang, C. Ambipolar Semiconducting Polymers with π-Spacer Linked Bis-Benzothiadiazole Blocks as Strong Accepting Units. Chem. Mater. 2014, 26, 4933–4942. [Google Scholar] [CrossRef]







| Mn | Mw | Đ 2 | C 3 | H 3 | N 3 | |
|---|---|---|---|---|---|---|
| (kDa) | (kDa) | (%) | (%) | (%) | ||
| PTVDPP-BSz | 22.82 | 49.43 | 2.16 | 73.29 | 8.56 | 5.67 |
| repeating unit 1 | - | - | - | 73.51 | 8.87 | 5.36 |
| Coating Speed (rpm) | Annealing Temperature (°C) | µmax 1 (cm2/(V s)) | µave 2 (cm2/(V s)) | Threshold Voltage (V) | On/Off Ratio |
|---|---|---|---|---|---|
| 3000 | 150 | 0.34 | 0.32 | 4.90 | 7.63 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Wang, S.; Chen, J.; Yi, Z. Design of Novel Functional Conductive Structures and Preparation of High-Hole-Mobility Polymer Transistors by Green Synthesis Using Acceptor–Donor–Acceptor Strategies. Polymers 2024, 16, 396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030396
Ren S, Wang S, Chen J, Yi Z. Design of Novel Functional Conductive Structures and Preparation of High-Hole-Mobility Polymer Transistors by Green Synthesis Using Acceptor–Donor–Acceptor Strategies. Polymers. 2024; 16(3):396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030396
Chicago/Turabian StyleRen, Shiwei, Sichun Wang, Jinyang Chen, and Zhengran Yi. 2024. "Design of Novel Functional Conductive Structures and Preparation of High-Hole-Mobility Polymer Transistors by Green Synthesis Using Acceptor–Donor–Acceptor Strategies" Polymers 16, no. 3: 396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030396