Synthesis, Characterization, Thermal Stability and Sensitivity Properties of New Energetic Polymers—PVTNP-g-GAPs Crosslinked Polymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
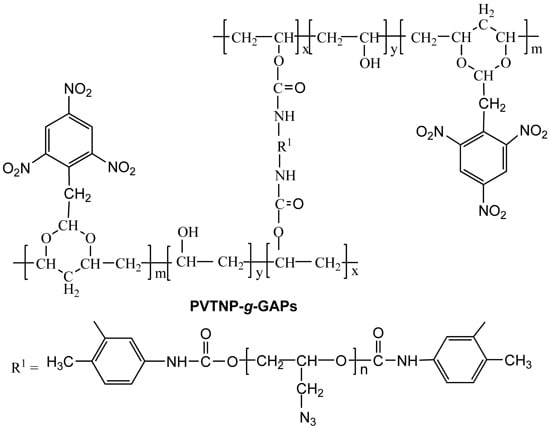
2.3. Synthesis

2.3.1. Synthesis of Poly(vinyl 2,4,6-trinitrophenylacetal) (PVTNP)
2.3.2. Synthesis of Poly(vinyl 2,4,6-trinitrophenylacetal)-g-1#polyglycidylazide (PVTNP-g-1#GAP)
2.3.3. Synthesis of Poly(vinyl 2,4,6-trinitrophenylacetal)-g-2#polyglycidylazide (PVTNP-g-2#GAP)
2.3.4. Synthesis of Poly(vinyl 2,4,6-trinitrophenylacetal)-g-3#polyglycidylazide (PVTNP-g-3#GAP)
3. Results and Discussion
3.1. Characterization of PVTNP-g-GAPs




3.2. Thermal Stability of PVTNP-g-GAPs




3.3. Sensitivity of PVTNP-g-GAPs
| Sample | Friction Sensitivity (%) | Impact Sensitivity (%) | H50 (cm) * |
|---|---|---|---|
| PVTNP-g-1#GAP | 100 | 76 | 43 |
| PVTNP-g-2#GAP | 100 | 96 | 42 |
| PVTNP-g-3#GAP | 100 | 64 | 48 |
| HMX [11] | 100 | 100 | 25 |
3.4. Compatibility Testing
| Creteria (ΔTp, °C) | Rating * | Single System |
|---|---|---|
| Less than or equal to 2 | A | Compatible or good compatibility |
| 3–5 | B | Slightly sensitized or moderate compatibility |
| 6–15 | C | Sensitized or poor compatibility |
| 15–above | D | Hazardous or bad compatibility |
| System | Maximum Exothermic Peak Temperature | |||
|---|---|---|---|---|
| Mixture System | Single System | Tp2 (°C) | Tp1 (°C) | ΔTp (°C) |
| PVTNP-g-3#GAP/TNT | PVTNP-g-3#GAP | 233 | 222 | −11 |
| PVTNP-g-3#GAP/HMX | PVTNP-g-3#GAP | 224 | 222 | −2 |
| PVTNP-g-3#GAP/RDX | PVTNP-g-3#GAP | 214 | 222 | 8 |
| PVTNP-g-3#GAP/FOX-7 | PVTNP-g-3#GAP | 225 | 222 | −3 |
| PVTNP-g-3#GAP/NTO | PVTNP-g-3#GAP | 221 | 222 | 1 |
| PVTNP-g-3#GAP/TATB | PVTNP-g-3#GAP | 226 | 222 | −4 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, H.J.; Dong, H.S.; Zhang, M. Problems and developments in composition B modification research. Chin J. Energ. Mater. 2001, 9, 183–186. [Google Scholar]
- Trzciński, W.; Cudziło, S.; Dyjak, S.; Nita, M. A comparison of the sensitivity and performance characteristics of melt-pour explosives with TNT and DNAN binder. Cent. Eur. J. Energ. Mater. 2014, 11, 443–455. [Google Scholar]
- Ravi, P.; Badgujar, D.M.; Gore, G.M.; Tewari, S.P.; Sikder, A.K. Review on melt cast explosives. Propell. Explos. Pyrot. 2011, 36, 393–403. [Google Scholar] [CrossRef]
- Colclough, M.E.; Desai, H.; Millar, R.W.; Paul, N.C.; Stewart, M.J.; Golding, P. Energetic polymers as binders in composite propellants and explosives. Polym. Adv. Technol. 1994, 5, 554–560. [Google Scholar]
- Eroğlu, M.S.; Hazer, B.; Güven, O.; Baysal, B.M. Preparation and thermal characterization of block copolymers by macroazonitriles having glycidyl azide and epichlorohydrin moieties. J. Appl. Polym. Sci. 1996, 60, 2141–2147. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Pethaiah, S.S.; Rajendran, S. Li-ion conduction in PVAc based polymer blend electrolytes for lithium battery applications. Mater. Chem. Phys. 2011, 129, 471–476. [Google Scholar] [CrossRef]
- Lin, M.J.; Ma, H.H.; Shen, Z.W.; Wan, X.Z. Effect of auminum fiber content on the underwater explosion performance of RDX-based explosives. Propell. Explos. Pyrot. 2014, 39, 230–235. [Google Scholar] [CrossRef]
- Chen, P.W.; Huang, F.L.; Ding, Y.S. Microstructure, deformation and failure of polymer bonded explosives. J. Mater. Sci. 2007, 42, 5272–5280. [Google Scholar] [CrossRef]
- Drodge, D.R.; Williamson, D.M. Understanding damage in polymer-bonded explosive composites. J. Mater. Sci. 2016, 51, 668–679. [Google Scholar] [CrossRef]
- Stanton, H.D.; Reed, J.R. Polymer Modified TNT Containing Explosives. U.S. Patent 4,445,948, 1 May 1984. [Google Scholar]
- Dong, H.S.; Zhou, F.F. Performance of High Explosives and Related Materials; Science Press: Beijing, China, 1989. [Google Scholar]
- Huang, H.J.; Dong, H.S.; Zhang, M.; Xi, Y. Research on modification of composition B with polymers (II). Chin. J. Energ. Mater. 2005, 13, 7–9. [Google Scholar]
- An, C.W.; Li, F.S.; Song, X.L.; Wang, Y.; Guo, X.D. Surface coating of RDX with a composite of TNT and an energetic-polymer and its safety investigation. Propell. Explos. Pyrot. 2009, 34, 400–405. [Google Scholar] [CrossRef]
- Eroğlu, M.S.; Güven, O. Characterization of network structure of poly (glycidyl azide) elastomers by swelling, solubility and mechanical measurements. Polymer 1998, 39, 1173–1176. [Google Scholar] [CrossRef]
- Kawamoto, A.M.; Holanda, J.A.S.; Barbieri, U.; Polacco, G.; Keicher, T.; Horst, K.; Kaiser, M. Synthesis and characterization of glycidyl azide-r-(3,3-bis(azidomethyl)oxetane) copolymers. Propell. Explos. Pyrot. 2008, 33, 365–372. [Google Scholar] [CrossRef]
- You, J.S.; Kweon, J.O.; Kang, S.C.; Noh, S.T. A kinetic study of thermal decomposition of glycidyl azide polymer (GAP)-based energetic thermoplastic polyurethanes. Macromol. Res. 2010, 18, 1226–1232. [Google Scholar] [CrossRef]
- Klapoetke, T.M.; Sproll, S.M. Investigation of nitrogen-rich energetic polymers based on alkylbridged bis-(1-methyl-tetrazolylhydrazines). J. Appl. Polym. Sci. 2010, 48, 122–127. [Google Scholar] [CrossRef]
- Jin, B.; Shen, J.; Peng, R.F.; Shu, Y.J.; Tan, B.S.; Chu, S.J.; Dong, H.S. Synthesis, characterization, thermal stability and sensitivity properties of the new energetic polymer through the azidoacetylation of poly(vinyl alcohol). Polym. Degrad. Stab. 2012, 97, 473–480. [Google Scholar] [CrossRef]
- Jin, B.; Dong, H.S.; Peng, R.F.; Shen, J.; Tan, B.S.; Chu, S.J. Synthesis and characterization of poly(vinyl 2,4,6-trinitrophenylacetal) as a new energetic binder. J. Appl. Polym. Sci. 2011, 122, 1643–1648. [Google Scholar] [CrossRef]
- Wang, J.Q.; Satoh, M. Preparation and salt-resistivity of poly(vinyl alcohol)-4-hydroxy phthalate hydrogels. Macromol. Res. 2011, 19, 468–475. [Google Scholar] [CrossRef]
- Reshmi, S.K.; Vijayalakshmi, K.P.; Thomas, D.; Arunan, E.; Reghunadhan, N.C.P. Glycidyl azide polymer crosslinked through triazoles by click chemistry: Curing, mechanical and thermal properties. Propell. Explos. Pyrot. 2013, 38, 525–532. [Google Scholar] [CrossRef]
- Nazri, N.A.M.; Lau, W.J.; Padaki, M.; Ismail, A.F. A facile modification approach for polyacrylonitrile-based UF hollow fiber membrane utilizing polyacrylonitrile-g-poly(vinyl alcohol) graft copolymer. J. Polym. Res. 2014, 21, 594. [Google Scholar] [CrossRef]
- Schmid, M.; Sängerlaub, S.; Miesbauer, O.; Verena, J.; Johannes, W.; Camelia, S.; Daniel, S.; Cornelia, S.; Klaus, N.; Kajetan, M. Water repellence and oxygen and water vapor barrier of PVOH-coated substrates before and after surface esterification. Polymers 2014, 6, 2764–2783. [Google Scholar] [CrossRef]
- Sanli, D.; Erkey, C. Silylation from supercritical carbon dioxide: A powerful technique for modification of surfaces. J. Mater. Sci. 2015, 50, 7159–7181. [Google Scholar] [CrossRef]
- Huang, T.; Jin, B.; Peng, R.F.; Chen, C.D.; Zheng, R.Z.; He, Y.; Chu, S.J. Synthesis and characterization of [60]fullerene-glycidyl azide polymer and its thermal decomposition. Polymers 2015, 7, 896–908. [Google Scholar] [CrossRef]
- Ye, H.; Chen, L.; Li, A.; Huang, L.L.; Zhang, Y.Z.; Li, Y.N.; Li, H. Alkali-responsive membrane prepared by grafting dimethylaminoethyl methacrylate onto ethylene vinyl alcohol copolymer membrane. J. Appl. Polym. Sci. 2015, 132, 41775. [Google Scholar] [CrossRef]
- Jin, B.; Shen, J.; Peng, R.F.; Shu, Y.J.; Chu, S.J.; Dong, H.S. Synthesis, characterization, and thermal stability properties of PVTNP-co-PVAA through the azidoacetylation of polyvinyl 2,4,6-trinitrophenylacetal. Macromol. Res. 2014, 22, 117–123. [Google Scholar] [CrossRef]
- Dimitry, O.I.; Abdeen, Z.I.; Ismail, E.A.; Saad, A.L.G. Preparation and properties of elastomeric polyurethane/organically modified montmorillonite nanocomposites. J. Polym. Res. 2010, 17, 801–813. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, M.P.; Raju, K.M. Synthesis, spectral and DSC analysis of glycidyl azide polymers containing different initiating diol units. J. Appl. Polym. Sci. 2004, 93, 2157–2163. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, K.M. Synthesis and characterization of low molecular weight azido polymers as high energetic plasticizers. Int. J. Polym. Anal. Charact. 2005, 9, 289–304. [Google Scholar] [CrossRef]
- Huang, T.; Jin, B.; Peng, R.F.; Chu, S.J. Synthesis and characterization of a new energetic plasticizer: Acyl-terminated GAP. Int. J. Polym. Anal. Charact. 2014, 19, 522–531. [Google Scholar] [CrossRef]
- Paul, C.J.; Nair, M.R.G.; Neelakantan, N.R.; Koshyc, P.; Idaged, B.B.; Bhelhekard, A.A. Segmented block copolymers of natural rubber and 1,3-butanediol–toluene diisocyanate oligomers. Polymer 1998, 39, 6861–6874. [Google Scholar] [CrossRef]
- Miyazaki, K.; Sato, H.; Kikuchi, S.; Nakatani, H. Dehydrochlorination of poly(vinyl chloride) modified with titanium dioxide/poly(ethylene oxide) based paint photocatalysts. J. Appl. Polym. Sci. 2014, 131, 40760. [Google Scholar] [CrossRef]
- Jung, J.H.; Lim, Y.G.; Lee, K.H.; Koo, B.T. Synthesis of glycidyl triazolyl polymers using click chemistry. Tetrahedron Lett. 2007, 48, 6442–6448. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Synthesis and explosive decomposition of polynitro[60]fullerene. Carbon 2013, 62, 413–421. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Luo, N.; Huang, M.; Jin, M.; Luo, Y.J. Thermal decomposition of energetic thermoplastic elastomers of poly(glycidyl nitrate). J. Appl. Polym. Sci. 2014, 131, 40965. [Google Scholar] [CrossRef]
- Eceiza, A.; Martin, M.D.; de La Caba, K.; Kortaberria, G.; Gabilondo, N.; Corcuera, M.A.; Mondragon, I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties. Polym. Eng. Sci. 2008, 48, 297–306. [Google Scholar] [CrossRef]
- Jin, B.; Peng, R.F.; Chu, S.J.; Huang, Y.M.; Wang, R. Study of the desensitizing effect of different [60]fullerene crystals on cyclotetramethylenetetranitramine (HMX). Propell. Explos. Pyrot. 2008, 33, 454–458. [Google Scholar] [CrossRef]
- Lee, J.S.; Jaw, K.S. Thermal decomposition properties and compatibility of CL-20, NTO with silocone rubber. J. Therm. Anal. Calorim. 2006, 85, 463–467. [Google Scholar] [CrossRef]
- Li, J.Z.; Zhao, F.Q.; Hu, R.Z. Compatibility study of 1,3,3-trinitroazetidine with some energetic components and inert materials. J. Therm. Anal. Calorim. 2006, 85, 779–784. [Google Scholar]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.; Shen, J.; Gou, X.; Peng, R.; Chu, S.; Dong, H. Synthesis, Characterization, Thermal Stability and Sensitivity Properties of New Energetic Polymers—PVTNP-g-GAPs Crosslinked Polymers. Polymers 2016, 8, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8010010
Jin B, Shen J, Gou X, Peng R, Chu S, Dong H. Synthesis, Characterization, Thermal Stability and Sensitivity Properties of New Energetic Polymers—PVTNP-g-GAPs Crosslinked Polymers. Polymers. 2016; 8(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8010010
Chicago/Turabian StyleJin, Bo, Juan Shen, Xiaoshuang Gou, Rufang Peng, Shijin Chu, and Haishan Dong. 2016. "Synthesis, Characterization, Thermal Stability and Sensitivity Properties of New Energetic Polymers—PVTNP-g-GAPs Crosslinked Polymers" Polymers 8, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8010010