Synthesis, Structures and Luminescence Properties of Metal-Organic Frameworks Based on Lithium-Lanthanide and Terephthalate
Abstract
:1. Introduction
2. Experimental Section
2.1. Hydrothermal Synthesis
2.2. Single-Crystal X-Ray Diffraction Studies
2.3. Powder X-Ray Diffraction Studies
2.4. Morphological Characterization
2.5. Infrared Spectra
2.6. Thermal Characterization
2.7. Photoluminescence Studies
3. Results
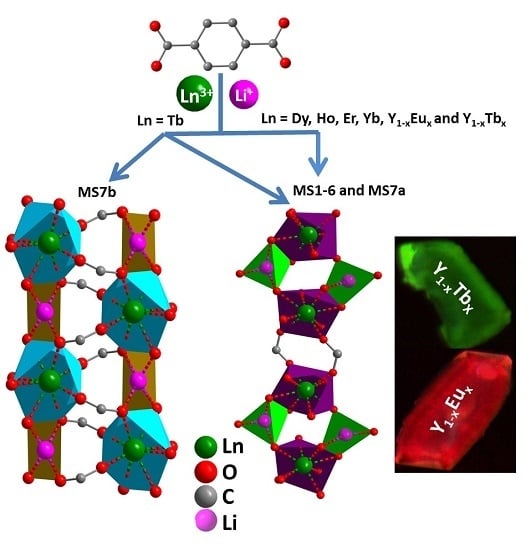
3.1. Description of the Crystal Structures
3.2. IR Analysis
3.3. Thermal Analysis
3.4. Potoluminescence Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xue, D.X.; Belmabkhout, Y.; Shekhah, O.; Jiang, H.; Adil, K.; Cairns, A.J.; Eddaoudi, M. Tunable Rare Earth fcu-MOF Platform: Access to Adsorption Kinetics Driven Gas/Vapor Separations via Pore Size Contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. [Google Scholar] [CrossRef] [PubMed]
- Alezi, D.; Peedikakkal, A.P.; Weseliński, Ł.J.; Guillerm, V.; Belmabkhout, Y.; Cairns, A.J.; Chen, Z.; Wojtas, Ł.; Eddaoudi, M. Quest for highly connected metal–organic framework platforms: Rare-Earth polynuclear clusters versatility meets net topology needs. J. Am. Chem. Soc. 2015, 137, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, W.S.; Kim, M.-B.; Kim, J.Y.; Bae, Y.-S.; Lee, J.; Jeong, N.C. A Chemical route to activation of open metal sites in the copper-based metal–organic framework materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-Y.; Li, Z.; Xiao, J.-X.; Deng, R.; Lin, P.-F.; Chen, R.-Q.; Liang, Y.-Q.; Guo, H.-F.; Liu, B.; Liu, J.-Q. Hydrostable and nitryl/methyl-functionalized metal–organic framework for drug delivery and highly selective CO2 adsorption. Inorg. Chem. 2015, 54, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Katz, M.J.; Wang, T.C.; Platero-Prats, A.E.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. High Efficiency Adsorption and removal of selenate and selenite from water using metal–organic frameworks. J. Am. Chem. Soc. 2015, 137, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; de Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; de Vos, D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef] [PubMed]
- Valvekens, P.; Vandichel, M.; Waroquier, M.; Van Speybroeck, V.; de Vos, D. Metal-dioxidoterephthalate MOFs of the MOF-74 type: Microporous basic catalysts with well-defined active sites. J. Catal. 2014, 317, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; et al. Synthesis Modulation as a Tool To Increase the catalytic activity of metal-organic frameworks: The unique case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chakraborty, A.; Maji, T.K. Lanthanide–organic frameworks for gas storage and as magneto-luminescent materials. Coord. Chem. Rev. 2014, 273–274, 139–164. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.M.; Amghouz, Z.; García-Granda, S.; García, J.R. A metal-organic framework assembled from Y(III), Li(I), and terephthalate: Hydrothermal synthesis, crystal structure, thermal decomposition and topological studies. Dalton Trans. 2014, 43, 5739–5746. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, M.S.M.; Amghouz, Z.; Fernández-Zapico, E.; García-Granda, S.; García, J.R. Metal-organic Frameworks assembled from lanthanide and 2,5-pyridinedicaboxylate with cubane-like [Ln4(OH)4] building Units. J. Solid State Chem. 2015, 229, 197–207. [Google Scholar] [CrossRef]
- Amghouz, Z.; García-Granda, S.; García, J.R.; Ferreira, R.A. S.; Mafra, L.; Carlos, L.D.; Rocha, J. Series of metal organic frameworks assembled from Ln(III), Na(I), and Chiral flexible-achiral rigid dicarboxylates exhibiting tunable UV–vis–IR light emission. Inorg. Chem. 2012, 51, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Wang, X.-S.; Cao, R. Microwave-assisted large scale synthesis of lanthanide metal–organic frameworks (Ln-MOFs), having a preferred conformation and photoluminescence properties. Dalton Trans. 2015, 44, 11954–11962. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Kido, J. Organo lanthanide metal complexes for electroluminescent materials. Chem. Rev. 2002, 102, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Singh-Wilmot, M.A.; Cahill, C.L.; Andrews, M.; Taylor, R. Isoreticular lanthanide metal-organic frameworks: Syntheses, structures and photoluminescence of a family of 3D phenylcarboxylates. Eur. J. Inorg. Chem. 2012, 2012, 4419–4426. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Hu, G.H.; Zhou, S.; Fan, R.; Yang, Y.; Xu, Y. A Series of lanthanide metal–organic frameworks with interesting adjustable photoluminescence constructed by helical chains. Chem. Eur. J. 2015, 21, 10391–10399. [Google Scholar] [CrossRef] [PubMed]
- Decadt, R.; Hecke, K.V.; Depla, D.; Leus, K.; Weinberger, D.; Driessche, I.V.; van der Voort, P.; Deun, R.V. Synthesis, crystal structures, and luminescence properties of carboxylate based rare-earth coordination polymers. Inorg. Chem. 2012, 51, 11623–11634. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Song, T.; Gao, J.; Cui, Y.; Yang, Y.; Wu, C.; Chen, B.; Qian, G. A highly sensitive mixed lanthanide metal–organic framework self-calibrated luminescent thermometer. J. Am. Chem. Soc. 2013, 135, 15559–15564. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Ryu, D.W.; Lee, J.W.; Yoon, J.H.; Koh, E.K.; Hong, C.S. Microporous lanthanide-organic frameworks with open metal sites: Unexpected sorption propensity and multifunctional properties. Inorg. Chem. 2010, 49, 4723–4725. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, S.Y.; Ma, J.F.; Liu, Y.Y.; Yu, Z.T. Syntheses, structures, photoluminescence, and gas adsorption of rare earth–organic frameworks based on a flexible tricarboxylate. Cryst. Growth Des. 2011, 11, 5469–5474. [Google Scholar] [CrossRef]
- Biswas, S.; Jena, H.S.; Goswami, S.; Sanda, S.; Konar, S. Synthesis and characterization of two lanthanide (Gd3+ and Dy3+)-based three-dimensional metal organic frameworks with squashed metallomacrocycle type building blocks and their magnetic, sorption, and fluorescence properties study. Cryst. Growth Des. 2014, 14, 1287–1295. [Google Scholar] [CrossRef]
- Nayak, S.; Nayek, H.P.; Pietzonka, C.; Novitchi, G.; Dehnen, S. A series of three-dimensional lanthanide MOFs: Observation of reversible structural changes controlled by solvent desorption–adsorption, and magnetic properties. J. Mol. Struct. 2011, 1004, 82–87. [Google Scholar] [CrossRef]
- Silva, P.; Cunha-Silva, L.; Silva, N.J. O.; Rocha, J.; Almeida Paz, F.A. Metal–organic frameworks assembled from erbium tetramers and 2,5-pyridinedicarboxylic acid. Cryst. Growth Des. 2013, 13, 2607–2617. [Google Scholar] [CrossRef]
- Shi, P.-F.; Chen, Z.; Xiong, G.; Shen, B.; Sun, J.-Z.; Cheng, P.; Zhao, B. Structures, luminescence, and magnetic properties of several three-dimensional lanthanide–organic frameworks comprising 4-carboxyphenoxy acetic acid. Cryst. Growth Des. 2012, 12, 5203–5210. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, J.; Liu, B.; Wang, L.; Ng, S.; Zhang, G.; Wang, J.; Shi, X.; Liu, Y. A series of lanthanide−organic frameworks based on 2-propyl-1h-imidazole-4,5-dicarboxylate and oxalate: Syntheses, structures, luminescence, and magnetic properties. Cryst. Growth Des. 2010, 10, 1399–1408. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.-F.; Hu, H.-M.; Wang, J.; An, R.; Dong, F.-X.; Yang, M.-L.; Xue, G.-L. Effect of pH on the construction of lead coordination polymers by the diverse coordination modes of sulfonate functionalized imidazophenanthroline derivative ligand. Polyhedron 2014, 81, 517–524. [Google Scholar] [CrossRef]
- Ma, L.-F.; Wang, L.-Y.; Lu, D.-H.; Batten, S.R.; Wang, J.-G. Structural variation from 1D to 3D: Effects of temperature and pH Value on the construction of Co(II)-H2tbip/bpp mixed ligands system. Cryst. Growth Des. 2009, 9, 1741–1749. [Google Scholar] [CrossRef]
- Gu, J.; Gao, Z.; Tang, Y. pH and Auxiliary ligand influence on the structural variations of 5(2′-carboxylphenyl) nicotate coordination polymers. Cryst. Growth Des. 2012, 12, 3312–3323. [Google Scholar] [CrossRef]
- Santra, A.; Bharadwaj, P.K. Solvent-induced structural diversity of partially fluorinated, stable Pb(II) metal–organic frameworks and their luminescence properties. Cryst. Growth Des. 2014, 14, 1476–1485. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Thuery, P.; Masci, B. Two- and Three-dimensional assemblies formed by alkali metal (Li+−Cs+) and Ba2+ ions with Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic acid. Cryst. Growth Des. 2010, 10, 4109–4117. [Google Scholar] [CrossRef]
- Horike, S.; Matsuda, R.; Tanaka, D.; Mizuno, M.; Endo, K.; Kitagawa, S. Immobilization of sodium ions on the pore surface of a porous coordination polymer. J. Am. Chem. Soc. 2006, 128, 4222–4223. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, J.-P.; Chen, X.-M.; Ng, S.W. Two highly-connected, chiral, porous coordination polymers featuring novel heptanuclear metal carboxylate clusters. Chem. Commun. 2008, 34, 4019–4021. [Google Scholar] [CrossRef] [PubMed]
- Tominaka, S.; Yeung, H.H.-M.; Henke, S.; Cheetham, A.K. Coordination environments and π-conjugation in dense lithium coordination polymers. CrystEngComm 2016, 18, 398–406. [Google Scholar] [CrossRef]
- Gou, L.; Zhang, H.-X.; Fan, X.-Y.; Li, D.-L. Lithium based coordination polymer as anode for Li-ion battery. Inorg. Chim. Acta. 2013, 394, 10–14. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Zhang, J.; Xu, F.; Sun, L.-X.; Zhang, T.; You, W.-S.; Zhao, Y.; Zeng, J.; Cao, Z.; Yang, D. Lithium-based 3D coordination polymer with hydrophilic structure for sensing of solvent molecules. Cryst. Growth Des. 2008, 8, 3127–3129. [Google Scholar] [CrossRef]
- Banerjee, D.; Kim, S.J.; Parise, J.B. Lithium based metal−organic framework with exceptional stability. Cryst. Growth Des. 2009, 9, 2500–2503. [Google Scholar] [CrossRef]
- Banerjee, D.; Borkowski, L.A.; Kim, S.J.; Parise, J.B. Synthesis and Structural characterization of lithium-based metal−organic frameworks. Cryst. Growth Des. 2009, 9, 4922–4926. [Google Scholar] [CrossRef]
- Banerjee, D.; Parise, J.B. Recent advances in s-block metal carboxylate networks. Cryst. Growth Des. 2011, 11, 4704–4720. [Google Scholar] [CrossRef]
- Peng, G.; Ma, L.; Cai, J.; Liang, L.; Deng, H.; Kostakis, G.E. Influence of alkali metal cation (Li(I), Na(I), K(I)) on the construction of chiral and achiral heterometallic coordination polymers. Cryst. Growth Des. 2011, 11, 2485–2492. [Google Scholar] [CrossRef]
- Frigoli, M.; El Osta, R.; Marrot, J.; Medina, M.E.; Walton, R.I.; Millange, F. Heterobimetallic sodium–lithium based metal–organic framework showing the β-cristobalite topology and having high permanent porosity. Eur. J. Inorg. Chem. 2013, 2013, 1138–1141. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; Han, S.S.; Goddard, W.A. High H2 uptake in Li-, Na-, K-metalated covalent organic frameworks and metal organic frameworks at 298 K. J. Phys. Chem. A 2012, 116, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Goddard, W.A. Lithium-Doped Metal-organic frameworks for reversible H2 storage at ambient temperature. J. Am. Chem. Soc. 2007, 129, 8422–8423. [Google Scholar] [CrossRef] [PubMed]
- Mulfort, K.L.; Hupp, J.T. Alkali metal cation effects on hydrogen uptake and binding in metal-organic frameworks. Inorg. Chem. 2008, 47, 7936–7938. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, A.; Araujo, C.M.; Srepusharawoot, P.; Ahuja, R. Li-decorated metal–organic framework 5: A route to achieving a suitable hydrogen storage medium. Proc. Natl. Acad. Sci. USA 2007, 104, 20173–20176. [Google Scholar] [CrossRef] [PubMed]
- Dalach, P.; Frost, H.; Snurr, R.Q.; Ellis, D.E. Enhanced hydrogen uptake and the electronic structure of lithium-doped metal-organic frameworks. J. Phys. Chem. C 2008, 112, 9278–9284. [Google Scholar] [CrossRef]
- Mavrandonakis, A.; Tylianakis, E.; Stubos, A.K.; Froudakis, G.E. Why Li doping in MOFs enhances H2 storage capacity? A multi-scale theoretical study. J. Phys. Chem. C 2008, 112, 7290–7294. [Google Scholar] [CrossRef]
- Klontzas, E.; Mavrandonakis, A.; Tylianakis, E.; Froudakis, G.E. Improving hydrogen storage capacity of MOF by functionalization of the organic linker with lithium atoms. Nano Lett. 2008, 8, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Kolmann, S.J.; Chan, B.; Jordan, M.J.T. Modelling the interaction of molecular hydrogen with lithium-doped hydrogen storage materials. Chem. Phys. Lett. 2008, 467, 126–130. [Google Scholar] [CrossRef]
- Wang, Lu.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Zou, F.; Chen, Y.-M.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Yu, Z. Metal organic frameworks derived hierarchical hollow NiO/Ni/Graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Dai, R.; Li, H.; Sun, W.; Wang, Y. Microwave hydrothermal synthesis of Ni-based metal−organic frameworks and their derived yolk−shell NiO for Li-ion storage and supported ammonia borane for hydrogen desorption. ACS Sustain. Chem. Eng. 2015, 3, 1830–1838. [Google Scholar] [CrossRef]
- Schmidt, S.; Sheptyakov, D.; Jumas, J.-C.; Medarde, M.; Benedek, P.; Novák, P.; Sallard, S.; Villevieille, C. Lithium iron methylenediphosphonate: A model material for neworganic−inorganic hybrid positive electrode materials for Li ion batteries. Chem. Mater. 2015, 27, 7889–7895. [Google Scholar] [CrossRef]
- Amghouz, Z.; Roces, L.; García-Granda, S.; García, J.R.; Souhail, B.; Mafra, L.; Shi, F.-N.; Rocha, J. Metal organic frameworks assembled from Y(III), Na(I), and chiral flexible-achiral rigid dicarboxylates. Inorg. Chem. 2010, 49, 7917–7926. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Li, L.; Ma, C.; Shen, Z.; Song, Y.; You, X. Structures and properties of porous coordination polymers based on lanthanide carboxylate building units. Inorg. Chem. 2010, 49, 10781–10787. [Google Scholar] [CrossRef] [PubMed]
- Black, C.A.; Costa, J.S.; Fu, W.T.; Massera, C.; Roubeau, O.; Teat, S.J.; Aromı, G.; Gamez, P.; Reedijk, J. 3-D lanthanide metal-organic frameworks: Structure, photoluminescence, and magnetism. Inorg. Chem. 2009, 48, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Zhang, J.; Sun, L.-X.; Xu, F.; You, W.-S.; Zhao, Y. Solvothermal synthesis and characterization of a lithium coordination polymer possessing a highly stable 3D network structure. Inorg. Chem. Commun. 2008, 11, 396–399. [Google Scholar] [CrossRef]
- Stein, I.; Ruschewitz, U. Poly[di-[mu]3-aqua-[mu]4-terephthalato-dirubidium]. Acta Crystallogr. Sect. E 2006, E62, m2116–m2118. [Google Scholar] [CrossRef]
- Stein, I.; Ruschewitz, U. Poly[di-[mu]3-aqua-[mu]4-terephthalato-dicaesium]. Acta Crystallogr. Sect. E 2007, E63, m382–m384. [Google Scholar] [CrossRef]
- Dale, S.H.; Elsegood, M.R.J. Poly[sodium(I)-[mu]6-hydrogen benzene-1,4-dicarboxylato]. Acta Crystallogr. Sect. C 2003, E59, m475–m477. [Google Scholar] [CrossRef]
- CrysAlis CCD; Version 1.171.32.37; release 24-10-2008 CrysAlis171. NET, compiled Oct 24, 2008, 09:44:38; Oxford Diffraction Ltd.: Abingdon, UK, 2008.
- CrysAlis RED; Version 1.171.32.37; release 24-10-2008 CrysAlis171. NET, compiled Oct 24, 2008, 09:44:38; Oxford Diffraction Ltd.: Abingdon, UK, 2008.
- Sheldrick, G.M. SHELXL-97, Program for Refinement of Crystal Structures; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Parkin, S.; Moezzi, B.; Hope, H. XABS2: An empirical absorption correction program. J. Appl. Crystallogr. 1995, 28, 53–56. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. Sect. A Fundam. Crystallogr. 1990, 46, C34. [Google Scholar]
- Spek, A.L. PLATON, a multipurpose crystallographic tool; Utrecht University: Utrecht, Netherlands, 1998. [Google Scholar]
- Brandenburg, K. DIAMOND; Version 3.1; Crystal Impact GbR: Bonn, Germany, 2007. [Google Scholar]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. Available online: http://www.topos.ssu.samara.ru (accessed on 20 January 2016).
- Blatov, V.A. Nanocluster analysis of intermetallic structures with the program package TOPOS. Struct. Chem. 2012, 23, 955–963. [Google Scholar] [CrossRef]
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ionsin Crystals; Wiley Interscience: New York, NY, USA, 1968. [Google Scholar]
- Debasu, M.L.; Ananias, D.; Rocha, J.; Malta, O.L.; Carlos, L.D. Energy-transfer from Gd(III) to Tb(III) in (Gd,Yb,Tb)PO4 nanocrystals. Phys. Chem. Chem. Phys. 2013, 15, 15565–15571. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, G.; Ren, H.; Li, Y.; Hewitt, I.J.; Qiu, S. The synthesis of multiwalled rare-earth phosphate nanomaterials using organophosphates with upconversion properties. Eur. J. Inorg. Chem. 2008, 2008, 2033–2037. [Google Scholar] [CrossRef]










| Identification Code | MS1 | MS2 | MS3 | MS4 | MS7b |
|---|---|---|---|---|---|
| Empirical formula | C16H14O11DyLi | C16H14O11HoLi | C16H14O11ErLi | C16H14O11YbLi | C16H8O8TbLi |
| Formula weight/g·mol−1 | 551.71 | 554.14 | 556.47 | 562.25 | 494.09 |
| Temperature/K | 293(2) | 296(2) | 293(2) | 293(2) | 293(2) |
| Wave length | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c | C2/c |
| Unit cell dimensions | |||||
| a(Å) | 11.6769(4) | 10.1727(7) | 10.1678(3) | 10.1110(2) | 13.611(1) |
| b(Å) | 16.1012(2) | 16.1068(8) | 16.1139(4) | 16.0630(3) | 26.1672(5) |
| c(Å) | 13.2509(5) | 13.228(1) | 13.2366(5) | 13.1703(2) | 6.7381(5) |
| β(°) | 132.240(6) | 122.037(5) | 122.113(2) | 122.193(2) | 136.14(1) |
| Cell volume/Å3 | 1,844.4(2) | 1,837.3(2) | 1,836.9(1) | 1,810.17(7) | 1,662.8(4) |
| Z | 4 | 4 | 4 | 4 | 4 |
| Calc. density/mg·m−3 | 1.987 | 2.003 | 2.012 | 2.063 | 1.974 |
| Absorption coefficient/mm−1 | 4.110 | 4.37 | 4.628 | 5.227 | 4.29 |
| F(000) | 1,068 | 1,072 | 1,076 | 1,084 | 944 |
| Crystal size/mm3 | 0.153 × 0.084 × 0.030 | 0.09 × 0.08 × 0.04 | 0.206 × 0.157 × 0.039 | 0.266 × 0.083 × 0.044 | 0.09 × 0.08 × 0.04 |
| Theta range for data collection/° | 3.27 to 31.81 | 3.28 to 31.16 | 3.27 to 31.26 | 3.29 to 31.39 | 3.3 to 31.4 |
| Index ranges | −17≤ h ≤ 17, −23 ≤ k ≤ 22, | −13 ≤ h ≤ 14, −22 ≤ k ≤ 23, | −14 ≤ h ≤ 14, −23 ≤ k ≤ 23, | −14 ≤ h ≤ 14, −23 ≤ k ≤ 23, | −19 ≤ h ≤ 19, −38 ≤ k ≤ 37, |
| −18 ≤ l ≤19 | −18 ≤ l ≤19 | −18 ≤ l ≤ 19 | −18 ≤ l ≤ 19 | −9 ≤ l ≤ 9 | |
| Reflections collected | 22,135 | 12,103 | 15,702 | 10,974 | 12,022 |
| Independent reflections | 6,297 | 5,932 | 5,982 | 5,968 | 2,761 |
| Completeness to theta = 67° | 99.80% | 99.04% | 99.45% | 99.80% | 99.78% |
| Absorption correction | multi-scan | multi-scan | multi-scan | multi-scan | multi-scan |
| Max. and min transmission | 0.933 and 0.883 | 0.836 and 0.678 | 0.836 and 0.424 | 1 and 0.861 | 0.838 and 0.682 |
| Refinement method | Full-matrix least squares on F2 | Full-matrix least squares on F2 | Full-matrix least squares on F2 | Full-matrix least squares on F2 | Full-matrix least squares on F2 |
| Data/restraints/parameters | 5,851/2/286 | 5,365/15/280 | 5,465/6/286 | 5,366/6/280 | 2,548/0/119 |
| Goodness -of -fit on F2 | 1.052 | 1.03 | 1.016 | 1.022 | 1.08 |
| Final R indices (I > 2sigma (I)) | R1 = 0.0288 (wR2 = 0.0682) | R1 = 0.055 (wR2 = 0.150) | R1 = 0.028 (wR2 = 0.061) | R1 = 0.0283 (wR2 = 0.057) | R1 = 0.0343 (wR2 = 0.0585) |
| R indices (all data) | R1 = 0.053 (wR2 = 0.0589) | R1 = 0.109 (wR2 = 0.236) | R1 = 0.048 (wR2 = 0.072) | R1 = 0.061 (wR2 = 0.0449) | R1 = 0.0436 (wR2 = 0.0624) |
| Largest diff. peak and hole/eÅ−3 | 0.545 and −1.335 | 2.74 and −3.21 | 0.950 and −1.452 | 0.736 and −0.103 | 1.70 and −1.60 |
| CCDC no. | 1444552 | 1444554 | 1444553 | 1444556 | 1444555 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbaky, M.S.M.; Amghouz, Z.; García-Granda, S.; García, J.R. Synthesis, Structures and Luminescence Properties of Metal-Organic Frameworks Based on Lithium-Lanthanide and Terephthalate. Polymers 2016, 8, 86. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030086
Abdelbaky MSM, Amghouz Z, García-Granda S, García JR. Synthesis, Structures and Luminescence Properties of Metal-Organic Frameworks Based on Lithium-Lanthanide and Terephthalate. Polymers. 2016; 8(3):86. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030086
Chicago/Turabian StyleAbdelbaky, Mohammed S. M., Zakariae Amghouz, Santiago García-Granda, and José R. García. 2016. "Synthesis, Structures and Luminescence Properties of Metal-Organic Frameworks Based on Lithium-Lanthanide and Terephthalate" Polymers 8, no. 3: 86. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8030086